pouring in distilled water and dried in a vacuum oven for
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1904
ISSN 2229-5518
Department of Chemistry, Faculty of Science, University of Damietta, Damietta 34517, Egypt.
ABSTRACT- Different concentrations of copolymer of N-(4-methoxy-2-methylphenyl)acrylamide(MA) with methyl methacrylate (MMA) were prepared and the reactivity ratio values of copolymerization were calculated using Microanalysis technique. Thermal analysis of the copolymers showed that the thermal stability are intermediate between poly(N-(4-methoxy-2-methylphenyl)acrylamide) (PMA) and poly(methyl methacrylate) (PMMA) homopolymers. Thermal degradation products of the PMA were identified by GC-MS techniques. It seems that the mechanism of degradation of PMA homopolymer is characterized by free radical formation followed by recombination along the backbone chain. The activation energies of the thermal degradation of the copolymers were calculated using Arrhenius relationship.
Keywords: N-(4-methoxy-2-methylphenyl)acrylamide, Thermal stability, Degradation and reactivity ratios.
* Correspondance Author: E-mail:elsonbatisch@yahoo.com; Tel.: +2 01060081581; Fax: +2 0572403868.
1Abstracted from her Ph.D. Thesis.
—————————— ——————————
Copolymerization is one of the most important means to improve the performance of thermal stability of polymers. Copolymers are extensively used in industrial processes, because their physical properties, such as elasticity, permeability, glass transition temperature (Tg) and solvent diffusion
kinetics can be varied within wide limits [1,2].
Knowledge of a copolymer’s composition is an important factor in the evaluation of its utility [3,4]. Controlling the polymer property parameters, such as copolymer composition, copolymer sequence distribution and molecular weight averages, is of particular importance in copolymerization processes.
This is because copolymer density and viscosity,
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1905
ISSN 2229-5518
which are two of the most important property measures used by polymer manufacturers, depend on these parameters [5]. Reactivity ratios are among the most important parameters for the composition equation of copolymers, which can offer information such as the relative reactivity of monomer pairs and help estimate the copolymer composition [3].
To calculate the polymerization rate or polymer
productivity and copolymer composition, monomer reactivity ratios must be known. The method which is used most often nowadays for estimating monomer reactivity ratios is to perform a low conversion copolymerization at various initial monomers feed
compositions. Subsequently, the copolymer
In this paper, homopolymes of N-(4-methoxy-2- methylphenyl) acrylamide (MA) and methyl methacrylate (MMA) and different composition of copolymers of N-(4-methoxy-2-methylphenyl) acrylamide and methyl methacrylate (MA-MMA) were prepared, so that the reactivity ratios might be determined using Microanalysis method. The thermal stability of the homopolymers and copolymers were examined. Thermal degradation of the PMA homopolymer was studied using GC-MS apparatus and the activation energies of the thermal degradation of the homopolymers and copolymers were calculated using Arrhenius relationship.
IJSER
composition is determined for each reaction [5]. Reactivity ratio values may be evaluated by various procedures: linear procedures, nonlinear procedures, and other copolymer composition equations [6,9].
Different concentrations of copolymer of N-(4- methoxy-2-methylphenyl) acrylamide with methyl acrylate were prepared and the reactivity ratio values of the copolymerization were calculated using 1H- NMR technique [10]. Thermal analyses of the copolymers showed that the stability of nitrogenated polymer has been improved by copolymerization with methyl acrylate. The activation energies of the thermal degradation of the copolymers were calculated using Arrhenius relationship.
Most of the thermal degradation results from free radical reactions initiated by bond breaking and depends on the relative strengths of the bonds that hold the molecules together. A large molecule will break apart and rearrange in a characteristic way [11].
Acryloyl chloride (AC) (Aldrich Chemical Co.,
Inc.) was used without further purification.It was stored below -18oC in a tightly glass-stoppered flask. 2,2'- Azobisisobutyronitrile (AIBN) (Aldrich Chemical Co., Inc.) was used as initiator for all polymerizations. It was purified by dissolving it in hot ethanol and filtering [12]. The solution was left to cool. The pure material was being collected by filtration and then dried. Methyl methacrylate (MMA) (BDH Chemical Ltd.), stabilized with 0.1% hydroquinone was washed with a small amount of sodium hydroxide solution, separated with a separating funnel, distilled on a vacuum line, dried over anhydrous sodium
sulphate and stored below -18oC. 2-Methyl-4-
methoxyaniline (Aldrich Chemical Co., Inc.) was used without further purification. All other chemicals and solvents were purified by standard procedures.
(N-(4-methoxy-2-methylphenyl)acrylamide) (MA) monomer was performed by the reaction of equimolar amounts of AC and 2-methyl-4-methoxyaniline in dry benzene until the evolution of hydrogen chloride ceased
forming a white powder of MA monomer. Poly(N-(4-
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1906
ISSN 2229-5518
methoxy-2-methylphenyl)acrylamide) (PMA) homopolymer was prepared by free radical initiation of MA using 0.1 w/v% AIBN as initiator and DMF as solvent and reflux for 6 hr. The polymer product was precipitated by
pouring in distilled water and dried in a vacuum oven for![]()
H2C CH
![]() +
+
![]()
![]()
C
O Cl
NH2
O
CH3
CH3
Amidation
![]()
![]()
![]()
H2C CH C
![]()
O ![]()

NH
CH3
several days at 40 0C.![]()
![]()
![]()
( C C )
H2 ![]() n
n
![]()
C
O ![]() O
O

![]()
NH
CH3
AIBN
CH3
Copolymers of MA with MMA were prepared using 0.2
O
CH3
w/v. % AIBN as free radical initiator and 50/50 (v/v) DMF as solvent. Different copolymer compositions of MA- MMA were prepared, so that the reactivity ratios might be determined. Polymerization was carried out to about 10%
Spectra were recorded on PyeUnicam SP 2000 spectrometry, for the homopolymers and copolymers in the form of KBr discs.
conversion. The polymers were precipitated by pouring into
a large excess of distilled water, filtered and dried in a
vacuum oven at 40oC for several days.
1H-NMR spectra were obtained using a Varian EM 390 90 MHz spectrometer with integration and 20 mg samples in dimethylsulphoxide (DMSO) solvent using tetramethylsilane (TMS) as internal reference.
Nitrogen content determination was performed by the
Microanalytical Unit at Cairo University.
TG measurements were made with a Mettler TG
3000 apparatus. Finely powdered (~10 mg) samples were heated at 10o/min in a dynamic nitrogen atmosphere (30 ml/min); the sample holder was boat-shaped, 10 mm x 5 mm x 2.5 mm deep and the temperature measuring thermocouple was placed 1 mm from the sample holder.
TG was also used for the determination of rates of
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1907
ISSN 2229-5518
degradation of the homopolymers and copolymers in the initial stages of decomposition. The activation energies
were obtained by the application of the Arrhenius equation.
Samples of ~ 50 mg were heated under vacuum from ambient temperature to 500 0C. The volatile degradation product was collected for qualitative analysis by GC-MS technique. A Saturn GC 3400 with fused quartz capillary column of 30m x 0.25mm coated methyl silicon under programmed heating condition from 60 to 200 0C was used for the identification of the condensable degradation products. The GC is interfaced with a Varian mass spectroscopy equipped with the standard electron impact
(E1) or chemical ionization (CI) sources and a DS 55 data
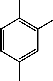
group of MA and carbonyl group of MMA in the copolymers, respectively [14]. The bands at 1600, 1545 and
1440 cm-1 are due to ν(C-H), ν(C=C) and ν(C-C) bonds
[15], respectively.
Fig. (2) shows the 1H-NMR spectrum of MA- MMA copolymers. The bands at ∂ 2.26 and 2.78-2.86 ppm are due to CH2 and CH protons of MA and MMA in the copolymers [16], the band at ∂ 8.16 ppm is due to –NH proton of MA in the copolymer [17]. The peak at δ 7.8 ppm due to phenyl protons of MA in the copolymer and
peak at 3.52 ppm is due to –OCH3 protons of MMA units in the copolymers and –OMe protons of MA units in the
copolymers.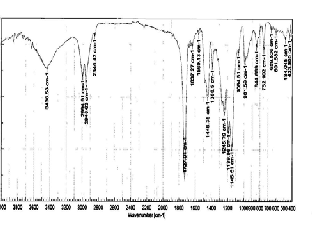
system scans from m/e 300 to 20 at a scan rate of 10 s/decade. Perflurokerosene (PFK) was used for computer calibration and the ion source was maintained at 200 0C. Accurate mass measurements of the CI mass spectra were performed at 1000 resolving power using PFK as internal
reference and by a computer interpolation data system.
The IR spectrum of PMA homopolymer shows a band at 1630 cm-1 is assigned to the antisymmetric stretching vibration of amidic carbonyl group. The bands at 1600, 1545 and 1440 cm-1 are assigned to ν(C-H), ν(C=C) and ν(C-C) bands, respectively [13]. The C-H in plane deformation in the region 1225-1045 cm-1, the ring
breathing at 995 and 1005 cm-1, the out-of-plan C-H
deformation vibration between 775 and 750 cm-1 and the C- C out-of-plan deformation at 500 cm-1 are assigned. The IR spectrum of MA-MMA copolymer as shown in Fig. (1) exhibit bands at 1637 and 1728 cm-1 assigned to
antisymmetric stretching vibration of the amidic carbonyl
Fig. 1. IR spectrum of MA-MMA copolymers
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1908
ISSN 2229-5518
Where,
F1 =
M 1 / M 2
![]()
M 1 / M 2 + 1
is the mole fraction of
n1
![]()
MMA (M1) in copolymers, f1 =
n1
+ n2
is the
mole fraction of M1 in feed and r 1 and r 2 are the reactivity ratios of MMA and MA, respectively. Fig. (4) is a plot of
f 2 (F
− 1)
f (1 − 2F )
1 1 versus
1 1 , and Fig. (5) is a
(1 − f1
) 2 F
f 2 (F
−1)
(1 − f1
)F1
f
(1 − 2F )
2 2
2 2
Fig. 2. 1H-NMR spectrum of MA-MMA copolymers
plot of
(1 − f 2
) 2 F
versus
(1 − f 2
)F2
where
F2 =
M 2 / M 1
![]()
M 2 / M 1 + 1
is the mole fraction of MA (M 2) in
copolymer and
f 2 =
n2
![]()
n1 + n2
is the mole fraction of M2
in feed. From the slope and intercept in Figures 4 and 5,
IJSER
Three different copolymers of MA-MMA with
40:1, 50:1 and 60:1 mole of MMA-MA covering the entire composition range between PMA and PMMA homopolymers were prepared, so the reactivity ratios might been be determined using microanalysis method. This method has already used for the determination of reactivity ratios for butyl acrylate - glysidyl methacrylate copolymer [18] and 2-methyl-N-1,3-thiazole-2-ylacrylamide- glycidylmethacrylate copolymer [19].
Fig. (3) shows the theoretical monomer compositions of the corresponding percentage nitrogen values. Percentage nitrogen data from microanalysis can then be inserted on the curves and the corresponding molar ratio read off from the abscissa.
By knowing the number of moles of the monomer mixture and the molar ratio of the copolymer, reactivity ratios can
be calculated by applying the following equation [20]:
reactivity ratio values for MA-MMA copolymer are:
r1 (MMA) = 20 and r 2 (MA) = 10.![]()
f1 (1 − 2F1 ) =
![]()
1 (F1 − 1) r + r
Fig. 3. Theoretical curve 0f nitrogen content versus copolymer ratio for
MMA-MA copolymers
(1 − f1
)F1
(1 − f1
) 2 F 1 2
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1909
ISSN 2229-5518
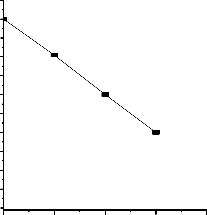
11
10
9
8
7
6
5
4
3
2
1
0
0.0 -0.1 -0.2 -0.3 -0.4
f 2(F -1)/(1-f )2F
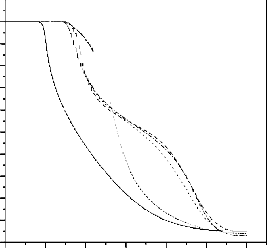
TG curves of PMA and PMMA homopolymers and MA-MMA copolymers are shown in Fig. (6) PMA homopolymer degrades in two stages. The first starts at
~110oC with a weight loss ~ 6%. The second stage starts at
~326oC with a weight loss of ~ 89%.
PMMA homopolymer shows two TG decomposition stages. The first starts at ~250oC with a weight loss ~ 53%. The second stage starts at ~375oC with a weight loss of ~
41%. There are three TG degradation stages for all the MA- MMA copolymers. The degradation temperature started at
~ 204, 206 and 207oC for the copolymers 40:1, 50:1 and
60:1 mole of MMA:MA. Table (1) represents the weight
f 2 (F
1 1
− 1)
1 1
f (1 − 2F )
loss percentage and the maximum rate of weight loss shown by derivative TG apparatus. TG curves of the
copolymers reveal that the stability of copolymers are
Fig. 4. Graph of 1 1 versus 1 1 for
(1 − f1
) 2 F
(1 − f1
)F1
intermediate between PMA and PMMA homopolymers.
MA-MMA copolymers.
The most clearly result is the increase of the
f2(1-2F2)/(1-f2)F2
25
20
15
10

thermal stability of PMA homopolymer and MA-MMA copolymers towards PMMA homopolymer. The effective activation energies for the thermal degradation of PMA and PMMA homopolymers and MA-MMA copolymers were determined from the temperature dependence of the chain rupture rate. The rate constant of the thermal degradation was plotted according to the Arrhenius relationship Fig. (7). Table (2) lists the activation energies of the homopolymers and copolymers, from which the values of activation
energy of the copolymers increasing from 45.73 to 241
5
0
0.0 -0.5 -1.0 -1.5 -2.0
f 2(F -1)/(1-f )2F
KJ/mol were obtained as the MMA concentration in the copolymer increases. It is clear that the rate of activation energies are in the same order as the stabilities.
2 2 2 2
Fig. 5. Graph of
f 2 (F − 1)
![]()
2
versus
f2 (1 − 2F2 )
(1 − f2 ) F2
![]()
(1 − f2 )F2
for MA-MMA copolymers.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1910
ISSN 2229-5518
110
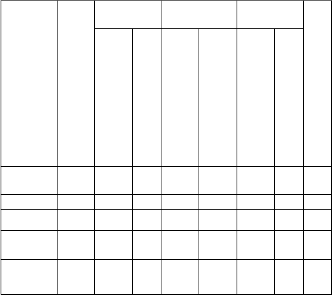
Table (1): Weight loss % of PMA and PMMA homopolymers and MA-MMA copolymers.
100
90
Polymer mole
Vol atili
First stage Second stage
Third Re stage m
MA:M
80 MA
70
60
50
40
30
zati
on Tem pera ture, oC
Tmax oC
W
t los s,
%
Tmax oC
Wt
Loss
, %
Tmax oC
W
t Lo ss,
%
ai
ni ng wt
%
aft
er
50
0o
C
20
10
0 100 200 300 400 500 600
Temprature(°C)
PMA 110 148 6 326 89 - - 4.
8
1:40 204 347 42 412 49 442 4 5
1:50 206 311 37 396 54 423 5 3
1:60 207 344 38 436 56 472 3 3
PMMA 250 350 53 500 41 - - 6
Fig. 6. TG curves for PMA and PMMA homopolymers and MA-MMA copolymers.
Table (2): Activation energies of the thermal degradation
of PMA and PMMA homopolymers and
MA-MMA copolymers.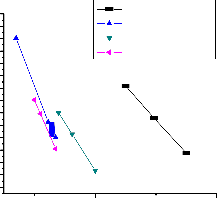
-1.0
-1.2
-1.4
-1.6
-1.8
-2.0
-2.2
-2.4
-2.6
-2.8
-3.0
-3.2
-3.4
-3.6
-3.8
PMA hompolymer
1 : 40 MA : MMA
1 : 50 MA : MMA
1 : 60 MA : MMA
2.0 2.4
1 / T X 10-3 K-1
Fig. 7. Arrhenius plots of the rate constants of degradation of PMA homopolymer and
MA-MMA copolymers.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1911
ISSN 2229-5518
50 mg of PMA homopolymer was heated under vacuum from ambient temperature to 500 oC. The volatile products of degradation were collected in a small
gas cell for identification by IR spectroscopy. Benzene,
Compounds 1 and 2 in the GC curve listed in Table 3 could be formed by abstraction of H by the radicals V and IV
and produce 4-methoxybenzenamine and 4-methoxy-2- methylbenzenamine.
CH3
aniline and ammonia were among the degradation products of PMA homopolymer. The liquid fractions
from the degradation of the homopolymer were injected
O
CH3
CH3
+ H ![]()
O
CH3
into the GC-MS apparatus . Fig. (8) shows the GC trace for the liquid products of degradation of PMA homopolymer at 500 oC . Table (3) gives the results of the degradation which were identified by mass spectroscopy. The various degradation products of PMA homopolymer indicate that the mechanism of degradation is characterized by the elimination of low molecular
weight radicals rather than monomer formation in the
V Compound 1
m/e 122
1-Methoxy-3-methylbenzene
NH2
![]()
NH
CH3 CH3
+ H ![]()
early stage of degradation, followed by random scission O
CH3
O
CH3
mechanism along the backbone chain. It seems that the breakdown of PMA homopolymer occurs mainly in the C- N bond producing the radicals:
IV Compound 2 m/e 137
4-Methoxy-2-methylbenzenamine![]()
![]()
![]()
![]()
![]()
![]()
CH2 CH
![]()
CH2 CH
Compound 3 could be formed by abstraction of H by the radical III.![]()
![]()
![]()
![]()
![]()
![]()
C C O
![]()
![]()
![]()
![]()
![]()
O O NH C C H
I II NH NH
CH3 CH3
![]()
+ H ![]()
![]()

NH O
![]()
C
CH3
O
CH3
![]()
O NH Me
Me
![]()
![]()
III Compound 3
m/e 165
N-(4-Methoxy-2-methylphenyl)formamide
OMe III
 Me
Me
OMe V
OMe IV
The suggested structure of compound 4 is formed by the reaction between two radicals V.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1912
ISSN 2229-5518
![]()
CH3
![]()
2 H3C O OMe
CH3
H3C
![]()
O CH3
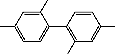
V Compound 4 m/e 242
1,1’-Dimethyl-3,3’-dimethoxydiphenyl
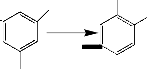
The mass spectrum of compound 5 is a termination reaction of the radicals IV and V.
H3C
NH
![]() +
+
OMe
OMe
CH3
CH3 H N
MeO

CH3
OMe
Fig. 8. GC curve of the liquid fraction of degradation of PMA
homopolymer
Table (3): GC-MS data of the liquid fraction of the degradation of PMA homopolymer
IV V Compound 5
BIis(4-metJhoxy-2-methylpShenyl)amine ER
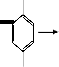
Compound 6 is formed by the dimerization of the radical
IV .
NH
![]()
![]()
CH3
H3C
H3C
2
H3C O
NH N O
CH3
OMe
IV Compound 6 m/e 272
1,2-Bis(4-methoxy-2-methylphenyl)hydrazine
The suggested structure of compound 7 is formed by the reaction between the radicals III and V .![]()
O
![]()
C
![]()
NH
H3C
+
OMe
OMe
![]()
CH3
![]()
H3C
O ![]() CH3
CH3
![]()
![]()
O HN C
H3C
![]()
O CH3
III V Compound 7 m/e 285
4-Methoxy-N-(4-methoxy-2-methylphenyl)-2-
methylbenzamide
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1913
ISSN 2229-5518
Different composition of copolymers of N-(4-Methoxy-
2-methylphenyl)acrylamide and methyl methacrylate (MA- MMA) were prepared and the reactivity ratios were determined using microanalysis method. Thermal degradation of poly(N-(4-methoxy-2- methylphenyl)acrylamide) (PMA) was studied and the
products of degradation were identified by GC-MS techniques. 1-Methoxy-3-methylbenzene, 4-Methoxy-2- methylbenzenamine, ammonia, N-(4-Methoxy-2- methylphenyl)formamide, 1,1’-Dimethyl-3,3’- dimethoxydiphenyl, Bis(4-methoxy-2-methylphenyl)amine,
1,2-Bis(4-methoxy-2-methylphenyl)hydrazine and 4- Methoxy-N-(4-methoxy-2-methylphenyl)-2- methylbenzamide were the main degradation products.
Accordingly, it seems that the mechanism of degradation of PMA is characterized by breaking down in the C-N bond producing low-molecular radicals. Combination of these radicals and random scission mechanism along the backbone chain are the main source of the degradation products.
REFERENCES
[8] Bajaj, P., Sen, K.,and Hajir, B.S., “Solution polymerization of acrylonitrile with vinyl acids in dimethylformamide,” J. Appl. Polym. Sci., 1996, 59,
1539–1550 .
[9] Soykan, C., Coskun, M., and Ahmedzade, M., “Synthesis
and characterization of phenacyl methacrylate- acrylonitrile copolymers and determination of monomer reactivity ratios,” Polym. Int., 2000, 49,479–
484 .
[10] Tirkistani, F. A. A., “Thermal stability of poly(N-[3-(5- amino-1,2,4-triazolo)]acrylamide) homopolymer and copolymer of N-[3-(5-amino-1,2,4-triazolo)]acrylamide with methyl acrylate,” Mans. J. Chem., 2008, 35, 195-
208.
[11] Sobeh, K.L., Baron, M., and Gonzales-Rodrigues, J., “Recent Trends and Developments in Pyrolysis–Gas Chromatography,” J.Chromatography, 2008, 1186,51-
66.
[12] Khairou, K.S. and Diab, M.A. “Thermal degradation of poly(acryloyl chloride) and copolymers of acryloyl chloride with metyl acrylate,” Polym. Deg. and Stab.,
1994,43, 329-333.
[13].Diab, M.A, “Thermal stability of poly homopolymer and copolymers of acryloyl chloride with methyl
IJSEacrylate,” ARctaPolymerica, 1994, 41, 731-738 .
[1] Liu, G., Zhang, L., Wang, Y., Zhao, P., “Studies on binary
copolymerization and glass transition temperatures of methyl methacrylate with ethyl methacrylate and n- butyl methacrylate,” J. Appl.Polym. Sci., 2009,114,
3939–3944.
[2] Saby-Dubreuil, A.C., Guerrier, B., Allain, C., “Glass transition induced by solvent desorption for statistical MMA/nBMA copolymers-Influence of copolymer composition,” Polymer, 2001, 42,1383–1391.
[3] Erol, I., Sen, O., Dedelioglu, A., Cifci, C., “Synthesis and characterization of novel fluorinecontaining methacrylate copolymers: Reactivity ratios, thermal properties, and antimicrobial activity,” J. Appl. Polym. Sci., 2009, 114, 3351–3359 .
[4] Hou, C., Liu, J., Ji, C., Ying, L., Sun, H., and Wang, C., “Monomer apparent reactivity ratios for acrylonitrile/methyl vinyl ketone copolymerization system,” J. Appl. Polym. Sci., 2006, 102,4045–4048 .
[5] Habibi, A., Farahani, E.V. , Semsarzadeh, M.A. , and Sadaghiani, K., “Monomer reactivity ratios for lauryl methacrylate-isobutyl methacrylate in bulk free radical copolymerization,” Polym. Int., 2003, 52, 1434–1443 .
[6] Hou, C., Ji, C., and Ying, L. , “Monomer apparent reactivity ratios for acrylonitrile/ammonium itaconate radical copolymerization systems,” J. Appl. Polym. Sci.,
2007,103, 3920–3923 .
[7] Miller, A., Szafko, J. , and Turska, E. , “Reactivity ratios for acrylonitrile-vinyl chloroacetate
copolymerization systems,” J. Polym. Sci., 1977,15, 51–63.
[14] Diab, M.A., El-Sonbati, A.Z., El-Sanabari, A.A. and
Taha, F.I., “Thermal stability of poly (2acrylamidobenzoic acid) homopolymer and polymer complexes of 2-acrylamidobenzoic acid with transition metal,” Acta Polymerica, 1990, 41, 351-359.
[15] El-Sonbati, A.Z. , Diab, M.A., Kotb, M.F., and Killa , H.M., “Structral chemistry of poly (2-acrylamido-1,2- diamidobenzene) complexes,” Bull. Soc. Chim. Fr.,
1991,128, 623-626 .
[16] Williams, D.H., and Fleming, I., "Spectroscopic method in organic chemistry," McGraw-Hill, London ,1966.
[17] Mochel, V.D., “Presented at a meeting of the division of rubber chemistry,” Amer. Chem. Soc., Montreal, Canada ,1967.
[18] Bakhshi, H., Zohuriaan-Mehr, M.J., Bouhendi, H., and Kabiri, K., “Emulsion Copolymerization of Butyl Acrylate and Glycidyl Methacrylate: Determination of Monomer Reactivity Ratios,” Iranian Polym. J., 2010
19 , 781-789 .
[19] Erol, I., Poyraz, B., Koroğlu, M. A. and Cifci, C., “Copolymerization of 2-methyl-N-1,3-thiazole-2- ylacrylamide with glycidyl methacrylate: synthesis, characterization, reactivity ratios and biological activity,” J. Polym. Res., 2009 16,19-28 .
[20] Billmeyer, F.W.Jr., "Textbook of polymer Science," New
York, Wiley Interscience, 1971, Chap 11, 328-354.
IJSER © 2013 http://www.ijser.org