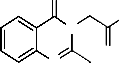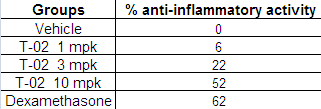International Journal of Scientific & Engineering Research, Volume 4, Issue 6 June 2013
ISSN 2229-5518 2928
The novel compound 2-(2-methyl-4-
oxoquinazolin-3(4H)-yl)-N-p-tolylacetamide (P- TOL) (T-02) attenuates inflammatory and noci- ceptive transmission in experimental animal
models of pain and inflammation
Siddiqui Masood Ahmed, Upasani Chandrashekhar Devidas
Abstract— Quinazoline-4(3H)-ones and its derivatives are versatile nitrogen heterocyclic compounds which have long been known as a promising class of biologically active compounds. Here we have investigated anti-inflammatory and anti-nociceptive potentials of some of the newly synthesized Quinazolines derivatives. Pharmacological activity was evaluated in the animal models of inflammation (Carrageenan induced paw edema, CFA induced inflammatory pain model) and Pain (formalin induced nociception) in male Sprague Dawley rats. We have evaluated T-02 compound and showed notably effective in reducing the not only carrageenan induced paw edema and CFA induced inflammatory pain but also formalin induced nociception. In carrageenan induced paw edema, CFA induced inflammatory pain and formalin induced nociception. W hen given orally, at similar doses produced significant reduction in paw volume, mechanical hyperalgesia (PWT -paw withdrawal threshold) and inhibition of nocifensive behavior (duration of paw licking and bitting) induced by subplanatar formalin injection. T-02 showed most prominent activity in both the animal models. T-02 at 1 mg/kg, 3 mg/kg, and 10 mg/kg significantly lowered nocifensive score in rats. The paw licking behavior in formalin test was more potently suppressed during the late phase (20–40 min, inflammatory) than in early phase (0–5 min, neurogenic) for T-02. Also; in CFA induced inflammatory pain model (T-02) at a dose of 1, 3 and 10 mg/kg significantly reduced the mechanical hyperalgesia (PW T -paw withdrawal threshold) show signs of anti- hyperalgesic activity and in carrageenan induce paw edema model 3 mg/kg, and 10 mg/kg (T-02) significantly reduced the edema reveal anti-inflammatory activity.
These results showed that Quinazoline derivatives produces antinociception possibly involving ion channels, which advantage further studies on its efficacy in more specific models of neuropathic pain such as Bennett and chung ‘s model of neuropathic pain
Key words--- Quinazoline, Pain and Inflammation, Carrageenan, Formalin, Paw edema, nociceptive pain, Nociception.
—————————— ——————————
1 Introduction
eterocyclic compounds are among the most frequently encountered scaffolds in drug discovery and pharceuti- cally industry. A heterocyclic core is propitious for vari-
ations of substitution pattern during Structure Activity Rela- tionship (SAR). Quinazolinone are tremendous reservoir for the synthesis of new chemical entities. The stability of the Quinazoline nucleus has inspired medicinal chemists to intro- duce many bioactive moieties to this nucleus to synthesize new potential medicinal agents. Quinazoline and quinazoli- none derivatives have continued to attract a widespread inter- est for a long time due to their diverse pharmacological activities such as antibacterial (1, 2), antitubecular (3), antifun- gal (4), antihyperglysemic (5), anti tumor (6).
In our research program we found that quinazolines and some
of condensed quinazolines exhibit potent activities like anal-
• Masood Ahmed Siddiqui. Department of Pharmacology, Faculty of pharmacy, Shri Neminath Jain Bramhacharyashram's Shriman Sureshdada Jain college
of pharmacy Jain gurukul, Chandwad, Nashik,423101 Maharashtra,India
masoodsiddiqui72@gmail.com
• Dr. Chandrashekhar Devidas Upasani Department of Pharmacology, Faculty of pharmacy, Shri Neminath Jain Bramhacharyashram's Shriman Sureshdada Jain college of pharmacy Jain gurukul, Chandwad, Nashik,423101 Maharash- tra,India
gesic, anti-inflammatory (7) and anticonvulsant (8). Quinazolin-4(3H) - ones with 2, 3-disubstitution is reported to possess significant analgesic, anti-inflammatory and anticon- vulsant activities (9, 10). Many diseases like arthritis, tem- pomadibular joint disorder, lower back pain, post-operative pain are typically associated with the pain such as hyperalge- sia and allodynia (11-14). It has been proved in many research publications that inflammatory pain can mark the changes in the neuronal plasticity due to peripheral sensitization of pri- mary sensory neuron in the dorsal root ganglion and subse- quently central sensitization in spinal cord (11).
Many Phlogistic agents like carrageenan, complete Freund’s
adjuvant, formalin, Zymosan, kaolin can cause tissue injury
inflammation and pain in rodents. Tissue injury due to any
agent (noxious or non-noxious stimulus) domino effect of in-
flammation and subsequently pain. Inflammatory mediators such as bradykinin, prostaglandins, prostacyclin, and nerve growth factor also some of the pro-inflammatory cytokines TNFα, IL-1β, IL-6 play important role in the peripheral sensi- tization of nociceptors. Most of the peripheral terminals of nociceptors express the receptors for almost all above men- tioned inflammatory mediators and activation of these recep-
IJSER © 2010 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6 June 2013
ISSN 2229-5518 2929
tors can cause hyperactivity of some of ion channels such as TRPA1 or TRPV1 or sodium, calcium ion channels via activa- tion of various kinases for instance protein kinase A (PKA), protein kinase C (PKC) and mitogen- activated protein kinases (MAPKs) (15).
Inflammatory diseases like rheumatoid arthritis (RA), osteoar- thritis (OA) inflammatory bowel disease (IBD), hepatitis, asthma are major cause of morbidity in humans. Early in- flammation is implicated in variety of disease such as cardio- vascular complication, diabetics, cancer (16). The treatment of inflammatory disease includes use of non steroidal anti- inflammatory drugs (NSAIDs).Non steroidal anti- inflammatory drug’s usually act by blocking the arachidonic acid metabolisms via cyclooxygenase and finally prostaglan- dins production. As Inflammation is protective phenomenon and synthesis of prostaglandin is key for the cytoprotection, but long -term synthesis of prostaglandin in human body can cause bleeding and ulcers and further renal complication as some of NSAIDs inhibit both the isoform COX-1 and COX-2 (17, 18).
Therefore, there is need to develop newer agents with more
potent analgesic and anti-inflammatory activities and with
lesser side effects and this is unmet therapeutic need. Quinazolines and condensed quinazolines exhibit diverse pharmacological activities. 2-phenyl-3-substituted quinazoline series has shown good analgesic and anti-inflammatory activi- ties (19), in the present study we aimed to synthesize some 2- methyl -3-substituted oxoquinazolin series for their analgesic and anti-inflammatory activities. The title compounds 2-(2- methyl-4-oxoquinazolin-3(4H)-yl)-N-p-tolylacetamide were prepared from an anthranilic acid or its derivative. Formation of 2-alkyl-4(3H) quinazolinone by condensation of anthranilic acid or substituted anthranilic acid and amides as designated in the Niementowski reaction (20).
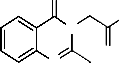

O
H
2. MATERIALS AND METHODS
2.1 Chemicals
Diclofenac Sodium (Sigma), Gabapentin (Fluorochem, Derby- shire, United Kingdom), Dexamethasone (Sigma), Carragee- nan (Sigma), CFA (Sigma).
2.2 Compounds
The test compound and standard were dissolved in 0.5%
Tween-80+0.5% carboxyl methyl cellulose solution.
2.3 Animals and experimental design
2.3.1 Animals
Male S.D Rats: 7-9 Weeks of age and body weight (210-250g) were procured from Laxmi Biofarms Pvt. Ltd. Ale Phata, Pu- ne. Animals were maintained at a constant temperature and had free access to food and drinking water in a 12 hr light/dark cycle with lights on from 06:30 to 18:30 h. Food and water were available ad libitum. All experimental procedures using animals were performed under the guidelines of our Institutional Animal Ethical Committee.
2.3.2 In vivo pharmacokinetic profile of T-02
Male S.D Rats: 7-9 Weeks of age and body weight (210-250g)
fasted for overnight were administered T-02 intravenously at 1
mg/kg dose and orally at 3 mg/kg dose. Blood samples fol-
lowing intravenous dosing were collected at 0.12 (7 min), 0.25
(15 min), 0.5 (30 min), 1, 2, 4, 8 and 24 h post dose (8 time points). Following oral dosing, blood samples were collected at 0.25 (15 min), 0.5 (30 min), 1, 2, 4, 8 and 24 h post dose (7 time points). Plasma samples were analyzed by LC-MS/MS following protein precipitation with acetonitrile containing internal standard.
2.3.3 In vivo efficacy of T-02 in inflammation (Carrageenan induced paw edema)
The initial hind paw volume of the Sprague–Dawley rats was determined volumetrically, 1% solution of carrageenan in sa- line (0.1 mL per rat) was injected s.c. into the plantar surface of the left hind paw 1 h after the test sample (1, 3, 10 mg/kg) had been administered orally. The paw volume was measured by
plethysmometer (# 7140) from Ugo Basile, Varese, Italy after
N
O N CH3
N CH3
3hr of carrageenan injection. Dexamethasone (30 mg/kg), an anti-inflammatory drug, was used as a positive control (24).
2.3.4 In vivo efficacy of T-02 in inflammatory pain (CFA In- duced inflammatory pain)
Fig. 1 T-02 (P-Tol) 2-(2-methyl-4-oxoquinazolin-3(4H)-yl)-N-p- tolylacetamide
There are no promising quinazolines which are in the market
in these NSAIDs criteria except few drugs like Proquazone
Afloqualone and Diproqualone etc (21, 22), fluproquazone (23)
has potent analgesic and anti inflammatory actions with less
gastric ulceration. So, present study was undertaken to ob- serve pharmacological effect of newly synthesized Quinazoli- none derivative in the animal models of inflammation (Carra- geenan induced paw edema, CFA induced inflammatory pain) and Pain (formalin induced nociception) in male Sprague Dawley rats.
So, the present study to the best of our search is the foot step
to report anti-inflammatory and anti-nociceptive potentials of
some of the newly synthesized Quinazolines derivatives.
CFA was injected Intra-plantar at concentration (75µg/150µL).
CFA induced mechanical hyperalgesia was quantified by Von
Frey test on day 2 (48h post injection of CFA). A cut off of <5.0 g was considered for selection of animals before treatment. The effect of test drug (1, 3, 10 mg/kg) and standard Gabapen- tin (150 mg/kg) administered orally on mechanical hyperalge- sia was measured at 60 min post treatment (25, 26).
Behavioral tests:
Measurement of mechanical hyperalgesia
Mechanical hyperalgesia was measured by using previously described up-down method (26) von Frey filaments (Bioseb, France) were used to assess mechanical hyperalgesia. The an- imals were placed in a plexiglass cage (16 x 24 x 14 cm) with a grid bottom and adapted for at least 10 minutes. Mechanical stimuli were generated by touching the plantar region of the
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6 June 2013
ISSN 2229-5518 2930
left hind paw of the rat with a continuous increas- ing/decreasing pressure changes by the pattern of (xoxoxo). For the paw withdrawal threshold the mean of two independ- ent measurements was calculated. Filaments used in experi- ment were 3.61, 3.84, 4.08, 4.31, 4.56, 4.74, 4.93 and 5.18 g. The values of the paw withdrawal thresholds were manually rec- orded and noted in result sheet (27).
2.3.5 In vivo efficacy of T-02 in nociceptive pain (Formalin induced nociception)
Animals were randomized by dose groups. On day of experi-
ment non fasted animals were weighed and dosed with test compounds (1, 3, 10 mg/kg) and standard Tramadol (40 mg/kg) orally 1hr. before formalin challenge. Formalin was injected s.c. into the dorsal lateral surface of the left hind paw and time spent licking and biting in seconds was recorded for each animal at 5 min interval for 40 min immediately after formalin injected (28).
2.4 Statistical analysis
One-way analysis of variance followed by Dunnett test and Two way analysis of variance followed by Bonferroni (ANO- VA; Graph Pad PRISM®, Version 4.0, San Diego, CA, USA) was applied to determine significant differences between the groups. A value of P<0.05 was considered significant. The pharmacokinetic parameters were calculated by a non- compartmental method with Win Nolin professional Version
4.1.
3. RESULTS
3.1 In vivo pharmacokinetic profile of T-02
The pharmacokinetics of T-02 (P-Tol) was evaluated in SD rats The PK profile of Test Compound T-02 (P-TOL) following PO administration, maximum plasma concentration (Cmax) was observed at 0.58 ±0.38 h (tmax) and the terminal half-life (t1/2, ß) was found to be 2.52±0.09 h. Following IV administration, elimination half life (t1/2, ß) was found to be 2.52±0.09 h and clearance was ~ 115.69±13.04 mL/min/Kg. The absolute oral bioavailability was 84 %.
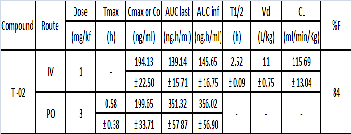
Table 1 Pharmacokinetic Parameters of T-02 (P-TOL) in Spra- gue Dawley Rat
3.2 Effect of T-02 on inflammation (Carrageenan induced paw edema)
The anti-inflammatory effects of tests compound T-02 (P-Tol)
were initially evaluated in rats with the goal of proving the anti-inflammatory property of test compound; we found that the oral administration of compound reduced significantly the carrageenan-induced paw edema. The results in (Fig 2) indi- cate that the administration of T-02 (P-Tol) (1, 3 and 10 mpk P.O.) 60 min before carrageenan reduced significantly the oe- dema at 3h after the carrageenan injection (Table 2). This result reinforces the idea that the test compound possesses peripher- al action, may relate to the arachidonic acid cascade.
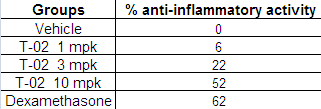
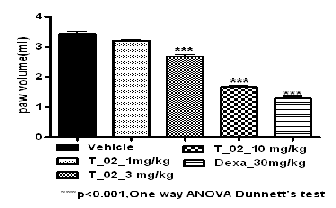
Table 2 Effect of T-02 on Carrageenan induced paw edema
Fig. 2 Effect of T-02 on Carrageenan induced paw edema
3.3 Effect of T-02 on inflammatory pain (CFA Induced in- flammatory pain)
In the present study, it has been demonstrated that acute oral
administration of the T-02 (P-Tol) compound, reduces the me- chanical hyperalgesia associated with an inflammatory pain model. In similar Gabapentin also reduced hyperalgesia. The results in (Fig 3) indicate that the administration of T-02 (P- Tol) (1, 3 and 10 mpk P.O.) reduced significantly CFA Induced Mechanical hyperalgesia (Table 3).
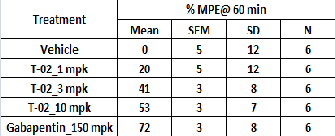
Table 3 Effect of T-02 on CFA induced mechanical hyperalge- sia
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6 June 2013
ISSN 2229-5518 2931
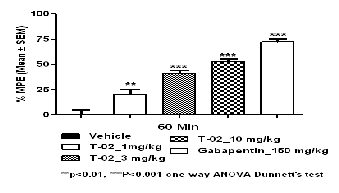
Fig. 3 Effect of T-02 on CFA induced mechanical hyperalgesia
3.4 Effect of T-02 on nociceptive pain (Formalin induced no- ciception)
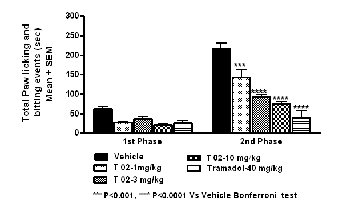
Oral administration of tests compound T-02 (P-Tol) prior for- malin injection decreased significantly the duration of nocifen- sive behaviors (licking and biting of injected paw) observed in phase II (Fig 4) in a dose-dependent fashion. Similarly, tra- madol, which is widely used to treat nociceptive pain, had significant effect in Phase II. The results presented here sup- port the hypothesis that formalin elicits pain-related behaviors by activating on primary afferent nociceptors. Additionally, these findings suggest that T-02 (P-Tol) blocks formalin- evoked responses in vivo through its inhibitory actions (Table
4).
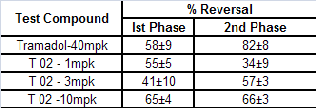
Table 4 Effect of T-02 on formalin induced nociception
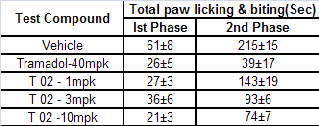
Table 5 Total paw licking and biting duration in sec.
Fig. 4 Total paw licking and biting duration in sec.
4. DISCUSSION
The present study strongly stated that the anti-inflammatory and anti-nociceptive activity of new derivative of Quinazoline
2-(2-methyl-4-oxoquinazolin-3(4H)-yl)-N-p-tolylacetamide.
In general, quinazoline derivatives are known to possess wide
range of activities. A specific activity depends on the substitu-
ent present at an appropriate position of quinazoline (29).
2-methyl -3-substituted oxoquinazolin derivatives with substi-
tution on benzene ring at position-3 possess remarkable anti-
inflammatory activity in carrageenan induced paw edema in
dose dependant manner. This edema depends on the partici-
pation of kinins and polymorphonuclear leucocytes with their
pro-inflammatory factors including prostaglandins. Develop- ment of edema in the paw of rat after carrageenan injection is a biphasic event. Initial phase observed during the first hour is attributed to the release of histamine and serotonin. The se- cond phase of edema is due to the release of prostaglandins, protease and lysosome. Carrageenan-induced edema is char- acterized by the presence of PGs and other compounds of slow reaction. COX-2 is an inducible isoform found in activat- ed inflammatory cells that generates prostanoid mediators of inflammation. The result of the present study indicates that 2- methyl -3-substituted oxoquinazolin derivatives and Diclo- fenac play a crucial role as protective factors against the carra- geenan-induced acute inflammation (30). Anti-inflammatory effect of 2-methyl -3-substituted oxoquinazolin could be due to the inhibition of COX-2 inflammatory mediator. This could be one of the possible mechanisms of 2-methyl -3-substituted oxoquinazolin derivative.
The further study was used to assess the effect of compound
on inflammatory pain. Injection of complete Freund’s adjuvant
(CFA) into a rat’s hind-paw provides a very good model in
order to study the mechanism of inflammatory pain and to
screen for anti-hyperalgesic drugs. CFA-induced mechanical hyperalgesia is mediated via peripheral activation of NMDA receptors and release of inflammatory mediator cycloxygenase (COX) after the injection of CFA. A 2-methyl -3- substituted oxoquinazolin derivative shows the good activity on inflam- matory pain in dose dependant manner. The exact mecha- nisms are not clear. The possible explanations might be due to the inhibition of inflammatory mediator as we have stated in earlier study or an antagonist of NMDA receptor (31).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6 June 2013
ISSN 2229-5518 2932
The anti-nociceptive effect of quinazoline derivative has been demonstrated by using Formalin induced nociception model. Oral administration of compound produced a dose-related antinociceptive effect when assessed in formalin induced noci- ception model. Formalin injection into the rat hindpaw initi- ates triphasic spontaneous nociceptive behaviors consisting of flinching, and licking and/or biting of the injected paw. The response to formalin is typically biphasic. The early phase of intense pain, named non-inflammatory pain which starts im- mediately after formalin injection, seems to be caused pre- dominantly by activation of C-fibers subsequent to peripheral stimulation. The late phase of moderate pain, named inflam- matory pain which starts about 20 min after formalin injection and lasts about 40 to 60 mins, appears to be caused by tissue and functional changes in the dorsal horn of the spinal cord and by local inflammation with a release of inflammatory me- diators (32). The antinociceptive effect of 2-methyl -3- substi- tuted oxoquinazolin derivative might be due the inhibitory action on inflammatory mediators.
To summarize the present work, we would like to state that
newly synthesized 2-methyl -3- substituted oxoquinazolin
derivative was found to be potent anti-inflammatory and an-
tinociceptive agent. The further studies should be initiated to know exact mechanism of action in different pain or diseased pain condition.
5. ACKNOWLEDGMENT
The authors would like to thank principal and staff of De- partment of Pharmacology, Faculty of pharmacy, Shri Nemi- nath Jain Bramhacharyashram's Shriman Sureshdada Jain Col- lege of pharmacy Jain gurukul, Chandwad, Nashik, India for providing the lab facilities.
6. REFERENCES
1. Jantova S, Stankovsky S, Spirkova K (2004) Biol Brati- slava 59:741
2. Tran TP, Ellsworth EL, Stier MA, Domagala JM,
Showalter HDH, Gracheck SJ,Shapiro MA, Joannides
TE, Singh R (2004) Bioorg Med Chem Lett 14:4405
3. Kunes J, Bazant J, Pour M, Waisser K, Slosarek M,
Janota J (2000) Il Farmaco 55:725
4. Dandia A, Singh R, Sarawgi P (2005) J Fluorine Chem
126:307
5. Ram VJ, Farhanullah, Tripathi BK, Srivastava AK
(2003) Bioorg Med Chem 11:2439
6. Bradly, D. S. Tetrahedron Lett. 2001, 42, 1851.
7. Alagarsamy V, Revathi R, Vijayakumar S, Ramseshu
KV. Synthesis and pharmacological investigation of
some novel 2,3-disubstituted quinazolin- 4(3H)-ones as analgesic and antiinflammatory agents. Pharmazie
2003; 58:4-8.
8. Alagarsamy V, Thangathirupathy A, Mandal SC, Ra-
jasekaran S, Vijayakumar S, Revathi R, et al. Pharma-
cological evaluation of 2-substituted (1,3,4) thiadia- zolo quinazolines. Indian J Pharm Sci 2006; 68: 108-11.
9. Bhalla M, Srivatava VK, Bhalla TN, Shanker K. Anti- inflammatory and analgesic activity of indolyl
IJSER © 2013
quinazolones and their congeners. Arzneimittel- forschung 1993; 43: 595-600.
10. Zappala` M, Grasso S, Micale N, Zuccala` G, Menniti FS, Ferreri G, et al. 1-Aryl-6,7-methylenedioxy-3H- quinazolin-4-ones as anticonvulsant agents. Bioorg Med Chem Lett 2003; 13: 4427-30.
11. Kuner R. Central mechanisms of pathological pain.
Nat Med. 2010; 16:1258–1266.
12. Hucho T, Levine JD. Signaling pathways in sensitiza-
tion: toward a nociceptor cell biology. Neuron. 2007;
55:365–376.
13. Brennan TJ, et al. Characterization of a rat model of incisional pain. Pain. 1996; 64:493–501.
14. Woolf CJ, Costigan M. Transcriptional and posttrans- lational plasticity and the generation of inflammatory pain. Proc Natl Acad Sci U S A. 1999; 96:7723–7730.
15. Ru-Rong Ji, Zhen-Zhong Xu, Gary Strichartz, and Charles N. Serhan Emerging Roles of Resolvins in the Resolution of Inflammation and Pain Trends Neuro- sci. 2011 November; 34(11): 599–609.
16. Hulya Uzkeser, Elif Cadirci, Zekai Halici, Fehmi
Odabasoglu, Beyzagul Polat, Tugba Nurcan Yuksel,3
Seda Ozaltin, and Fadime Atalay Anti-Inflammatory and Antinociceptive Effects of Salbutamol on Acute and Chronic Models of Inflammation in Rats: In- volvement of an Antioxidant Mechanism Mediators of Inflammation Volume 2012, Article ID 438912, 10 pages
17. Rainsford, K.D., 2007. Anti-inflammatory drugs in the
21st century. Sub-cellular Biochemistry 42, 3–27.
18. Tapiero, H., Ba, G.N., Couvreur, P., Tew, K.D., 2002.
Polyunsaturated fatty acids (PUFA) and eicosanoids
in human health and pathologies. Biomedicine & Pharmacotherapy 56, 215–222.
19. Alagarsamy V., Raja Salomon V., Vanikavitha G., Paluchamy V.,Ravichandran M., Arnald Sujin A., Thangathiruppathy A., Amuthalakshmi S., Revathi
R., Biol. Pharm. Bull., 25, 1432—1435 (2002).
20. Niementowski V. J. Prakt. Chem. 1895;51:564.
21. Tani. J, J. Med. Chem., 22:95. (1979).
22. Stephen P. Clissold, Rosemary Beresford. Proqua-
zone Drugs May 1987, Volume 33, Issue 5, pp 478-502
23. Ochiai T, Ishida R, Pharmacological studies on 6-
amino- 2-fluoromethyl- 3-(Otolyl)-4(3H)- quinazoli- none (afloqualone),a new centrally acting muscle re- laxant. (II) Effects on the spinal reflex potential andthe rigidity. Japanese Journal of Pharmacology, 32(3):427-
38, (1982).
24. H. Gerhard Vogel .Drug Discovery and Evaluation Pharmacological Assays Second Completely Revised, Updated, and Enlarged Edition Springer-Verlag Ber- lin Heidelberg 2002
25. S. S. Negus, T. W. Vanderah, M. R. Brandt, E. J. Bilsky,
L. Becerra, and D. Borsook, Preclinical Assessment of Candidate Analgesic Drugs: Recent Advances and Future Challenges J Pharmacol Exp Ther. 2006 No- vember ; 319(2): 507–514.
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6 June 2013
ISSN 2229-5518 2933
26. Chaplan SR, Bach FW,Pogrel JW,Chung JM,Yaksh TL, Quantitative assessment of tactile allodynia in the rat paw,Journal of Neuroscience Methods (53)1994, 55-63.
27. Bennett GJ and Xie YK. A peripheral mononeuropa- thy in rat that produces disorders of pain sensation like those seen in man. Pain, 1988; 33: 87-107.
28. Tjolsen, A., Berge, O.-G., Hunskaar, S., Rosland, J.H., Hole, K., The formalin test: an evaluation of the method. Pain 1992, 51, 5–17.
29. P. Mani chandrika, A. Raghu ram rao, B. Narsaiah
an d M . Bh ag aw an ra ju q u in azolin e d er iv ativ es w ith
potent Anti-inflammatory and anti-allergic Activities
Int. J. Chem. Sci.: 6(3), 2008, 1119-1146
30. Parmar Namita, Rawat Mukesh, Kumar Tirath Eval-
uation Of Anti-Inflammatory Potential Of Kigelia
Pinnata Leaf Extract In Wistar Rats. Journal of phar-
maceutical biology, 2(1), 2012, 35-39.
31. Cheng Huanga,, Zhi-Ping Hu, Hua Long, Yu-Shun
Shi , Ji-Sheng Hana, You Wan Attenuation of mechan-
ical but not thermal hyperalgesia by electroacupunc-
ture with the involvement of opioids in rat model of
chronic inflammatory pain. Brain Research Bulletin 63
(2004) 99–103
32. Fabiana Regina Nonato, Tais Adelita Almeida Barros,
Angélica Maria Lucchese, Carlos Eduardo Cordeiro
Oliveira, Ricardo Ribeiro dos Santos, Milena Botelho
Pereira Soares, Cristiane Flora Villarreal Antiinflam-
matory and antinociceptive activities of Blechnum oc-
cidentale L. extract Journal of Ethnopharmacology
125 (2009) 102–107
IJSER © 2013 http://www.ijser.org