Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h, Vo lume 3, Issue 1, January -2012 1
ISS N 2229-5518
The formation of Passivation in internally oxidized
Ag-based alloys
Ph.D.Ph.D. Jožica Bezjak A. Professor
—————————— ——————————
The passivation is a phenomenon wher e at certain depth fr om the sur face of the investigated material an oxide barr ier is formed. The for med barr ier is thick enough that further pr ogr ess of oxidation thr ough the material is not possible any mor e. In internally oxidised alloys the passivation has been obser ved for cer tain conditions of concentration of main element, partial pr essur e and temperatur e of oxidation[1 to 8]. W e attempt to study the inhibition of passivation by microalloying. W ith the addition of micr oalloying elements (0.001 to 0.5 mass%) with very high fr ee formation ener gy of oxides, r egarding the oxide of the main alloying element, we tr ied to inhibit this phenomenon and to enable an undisturbed pr ocess of oxidation, as w ell as a gr owth of internal oxidation zone.
Our pr evious investigations[6 to 8] established that Mg, Si, Ti, Al, Zr , Be ar e impor tant micr oalloying elements for Ag-Zn, Ag-In and Ag-Sn alloys. These microalloying elements have lar ge affinity to oxygen and ther efor e oxidise at low er par tial pr essur es (concentr ations) of oxygen in silver than the main alloying elements (Zn, In, Sn etc.). Their oxides form the nuclei for the gr owth of oxides of the main alloying element.
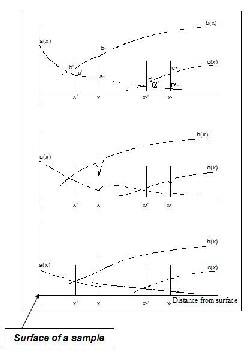
Ther efor e, by their distr ibution in the metal matr ix, the distr ibution of the main alloying element oxide is also determined (Fig. 1)[1,2].
_ _
Ph.D.Ph.D. J ožica Be zja k A. Professor, Unive rs ity of Primors ka ,
PEF, Pres ide nt of Associa tion of Technica l
Crea tive ness Educa tors Slovenia , jozica .bezja k2@gma il.com
IJSER © 2012 http:// www.ijser.org
Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h, Vo lume 3, Issue 1, January -2012 2
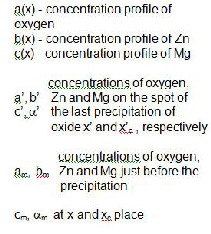
ISS N 2229-5518
The scheme of the concentration profile of oxygen and alloying elements at the beginning of the internal oxidation zone.
This heter ogeneous nucleation is very effective at the defined concentration interval of a micr oalloying element.
The aim of our r esear ch w as to analyse the inhibition mechanism of the formation of passivation in the Ag-Zn alloys by changing the fraction of main alloying element, as w ell as the fr action of micr oalloying element (Mg) and to estimate the range of concentrations at which the micr oalloying element Mg is still effective as a modificator .
The differ ent alloys (Table 1) w er e pr epar ed in evacuated (up to 10-2 bar ) quar tz tubes in an induction fur nace. After 30 hours of homogenisation and annea ling at 800 oC the alloys w er e hot for ged and r ecrystallization annealed. Finally, they w er e cold r olled into 3 mm thick stripes, w hich w er e then gr ound and etched in 10 % solution of HNO3 .
The tests of internal oxidation kinetics w er e made at differ ent temperatur es (in the temperatur e r ange between 750 oC and
850 oC), and at differ ent time (fr om 5 to 140 hour s) in a tube furnace, w her e air flow cir culation was assur ed.
In the most cases, the oxidising atmospher e was air . How ever , some tests, in the oxidising atmospher e of oxygen (w ith par tial pr essur e of 1.01105 Pa) w er e also per formed.
Micr ostructural changes in the zone of internal oxidation wer e
obser ved by optical (Leitz Wetzlar , Germany and Nikon Micr ophot –FXA, Japan) and scanning electr on micr oscopy (SEM/W DX analyser Jeol JSM 840 A). Volume and ener gy changes, as well as the pr ocess of inter nal oxidation wer e measur ed by thermogravimetr ic analysis (TGA, Mettler TG 50,
IJSER © 2012 http:// www.ijser.org
Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h, Vo lume 3, Issue 1, January -2012 3
ISS N 2229-5518
Germany). Applying the Wagner ’s theory*1 to 3+, the changes
of system kinetics wer e calculated.
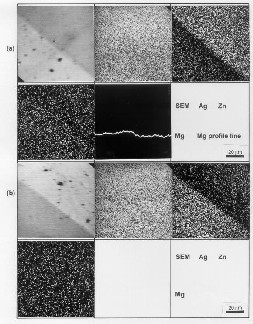
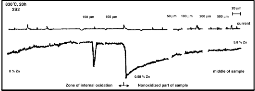
The distributions of Ag, Mg, and Zn in the r egion of internal oxidation zone and in the non-oxidised part of investigated alloys w er e observed (Fig. 2) and analysed by the electr on pr obe X-ray micr oanalyzer (EPMA; Jeol JXA 3A).
and to the middle part of the non-oxidised sample (obtained by
EPMA profile analysis; Jeol JXA 3A).
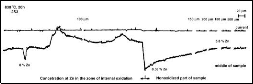
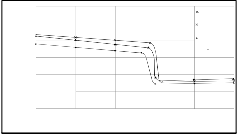
Fig. 3b Concentration profile of Zn in the sample of 2S3 alloy, microalloyed with 0.065% Mg, through the internal oxidation zone and part of the non-oxidised sample towards the middle of the sample (obtained by EPMA pr ofile analysis; Jeol JXA 3A).
The r esults of measur ements in the zone of inter nal oxidation and at the beginning show that the average concentrations of Zn ar e significantly higher than its starting concentr ation in the alloy. In the 2S3 alloy, the spots w ith slightly higher concentrations at crystal boundaries and at other defects in the alloy w er e observed w hich is the evidence of an impr oper concentration of microalloying element Mg. The measur ements of micr ohar dness also confirmed the measur ed values of concentration profiles (see Figs 4a and b) because the hardness is incr eased w ith incr eased content of oxide particles.
inte rnal oxidation zone and nonoxidised alloy; 830 oC/20 h/air, (a)
2S2 and (b) 2S3 alloys (obtained by EPMA profile and mapping
analysis; Jeol JXA 3A).
300
250
200
150
100
50
A
B B B
Ag-Zn 6.3%
Ag-Zn<0.002% Mg
Ag-Zn-0.51% Mg
830 C, 20 h , ai r A- complete passivation B- - partial passivation
In the front of the internal oxidation zone a stripe with lower
0
200
400 600 800
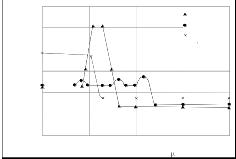
concentration of Zn can be noticed. This is evident fro m the concentration profile of Zn in the internal oxidation zone (Figs 3a and b). The differences in the concentration, as well as a distribution of Mg in both studied alloys were also determined.
x - distance from surface in( m)
300
250
200
150
100
50
Ag-Zn-0.29% Mg Ag-Zn-0.21% Mg Ag-Zn-0.065% Mg
830 C, 20h, ai r
0
200
400 600 800
microalloyed with 0.21% Mg, through the internal oxidation zone
y - distance from surface (m)
IJSER © 2012 http:// www.ijser.org
Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h, Vo lume 3, Issue 1, January -2012 4
ISS N 2229-5518
The rate of inter nal oxidation zone displacement (Fig. 5) and the mass changes of inter nally oxidised AgZnMg alloys measur ed by TGA w er e used to determine the r eaction kinetics of the inter nal oxidation.
Ther e ar e sever al equations in the literatur e[1 to 2] how to calculate the distance of inter nal oxidation zone fr om the sample’s sur face. The kinetics constant for the parab olic gr owth of the internal oxidation zone was calculated by the help of exper imental TGA data of isothermal measur ements. The equation:
(X)2 = Kt
(1)
was adapted as follows:
(m)2 = Kt
(2)
wher e in the equations (1) and (2) ar e:
x ….. depth of inter nal oxidation zone (m)
K ….. kinetics constant of inter nal oxidation (m/s2)
t ….. oxidation time

m …. mass of for med oxide
Ea ….. activation ener gy (for volume diffusion of oxygen)
R …… gas constant (8.31 kJ/kmolK)
T ….... ab solute temperatur e (K)
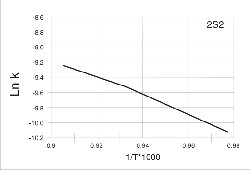
Taking into account the dependence of logar ithm of the r eaction rate constant from the r ecipr ocal value of the temperatur e (Fig. 6, Table 2), the value of Ea for the alloy w ith optimal composition (2S2) was deter mined: Ea = 103 4,8 kJ/mol
8
7
Table 2 Logarithmic values of reaction constant rate of 2S2 alloy
5
4
3
2
1
0 1 4 9 16 25 36 49 64 91 100 time (h)
The activation ener gy was der ived with the Arrhenius equation[2]:
ln K = ln K0 - Ea / RT (3)
wher e in the equation (3) ar e:
K0 ….. parametr ic constant of internal oxidation (m/s 2)
4 DISCUSSION
The r esults of kinetics, the investigations with electr on pr obe microanalyzer and optical micr oscopy, as w ell as the measur ements of hardness confir med the corr ect selection of alloys. The alloying with Mg in the quantities of 0.001 to 0.25 mass% the passivation w as inhibited, and undisturbed gr owth of internal oxidation zone was achieved.
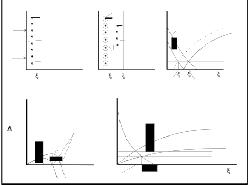
Our investigations pr oved that the inocculation ability depends on the discr epancy of fr ee ener gy of oxide formation and crystallogr aphic similarities of oxides. And because the oxide with high fr ee ener gy of formation is formed much ear lier than the oxide w ith low er fr ee ener gy of formation, it can serves as a nucleus (Figs 7a to d). At this kind of inocculation char acter istic for ternary alloys, ther e ar e two
IJSER © 2012 http:// www.ijser.org
Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h, Vo lume 3, Issue 1, January -2012 5
ISS N 2229-5518
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
kinds of oxides for med at differ ent times at the determined location. The oxide with high fr ee formation ener gy forms ear lier and its par ticles ar e always thinner and better disper sed (Fig. 7a). ZnO later form the pr ecipitates on them (Fig. 7b). Thus pr imary thin par ticles in our Zn oxides alloys ser ve as heter ogeneous nuclei wh ich makes the pr ecipitation of secondary oxides easier .![]()
![]()
![]()
![]()
![]()
t1
![]()
![]()
![]()
![]()
t2 t3
![]()
![]()
![]()
![]()
![]()
![]()
![]()
x1 < x 2 = x 3
![]()
![]()
![]()
a)
MgO
O 2
[O]
ZnO MgO
MgO
Ag - Zn
b) c)
[O]
[Me] No (p=1 bar)
No (p=0.2 bar )
[Zn ]
[Zn ]
sur fa ce of alloy
Ag - Zn - Mg
Ag - Zn
[O]
Ag - Zn - Mg
n - 1 n
TP ([ZnO]x [O])
TP…. solubility product
Depht of
1 1 2
precipitat i on of ZnO precipitat i on
internal
at po 2 = 0.2 bar
at po = 1 bar
oxid.zone
d) e)
G
r * MgO
ZnO
r *
ZnO
[O]
O
[Me]
TP(ZnxO)
N Zn
N
Fr om the micr ostr uctur e of inter nal oxidation zone shown in metallographic images (see Figs 10 to 15), it is evident that the
MgO
r
Zn O prec ipi tation MgO pr ecipitation
Mg
TP(MgxO)
low est concentr ation limit of the micr oalloying element Mg,
which alr eady acts as an inoculant, is approximately 0.001

The inocculation also causes the set of changes. These changes depend on the size, shape, distr ibution and disper sion of oxides of the main alloying element in the alloy. Compar ing the pr ecipitation of oxides in binary alloys (Fig. 8) w ith the pr ecipitation of oxides in modified ter nary alloys (Figs 9a to c and Fig. 10), one can conclude that at the latter the distribution of oxide particles is much mor e unifor m and r egular and ther efor e totally inhibits the passivation.
x1 > x2 > x 3
mass% and the highest concentr ation limit is appr oximately
0.25 mass%. The addition of Mg above upper limit incr eases the fraction of defect microstr uctural elements in the internally oxidised zone and thus it alone pr ovokes partial or even total passivation.
Fig. 10 Optical microscope micrograph of Internally oxidised zone in the 2S1 alloy (left), and in the 2S2 alloy (right); 830 0C/20h/air; left: occurrence of passivation, right: no passivation, larger depth of
y
1 y2 y3
x1
y1 < y2 < y 3
t1 < t2 < t 3
int. oxidation.
x2 x3
t1 surface of alloy
t2 surface of alloy
t3 surface of alloy
830 0C and at different times of oxidation.
IJSER © 2012 http:// www.ijser.org
Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h, Vo lume 3, Issue 1, January -2012 6
ISS N 2229-5518

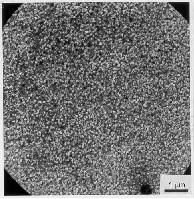
Fig. 14 Optical microscope micrograph of inte rnally oxidised zone in the 3S1 (left) and 2S2 alloy (right); 830 oC/20 h/air; larger depth of inte rnal oxidation because of the appropriate concentration of Mg .
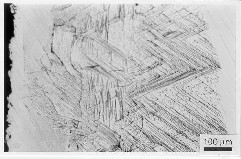
Fig. 11 Optical microscope micrograph of inte rnally oxidised zone in the 3S3 alloy (right) and in the 3S2 alloy (left); 830 oC/20 h/air; only partial passivation is noticed, complete passivation has not happened still.
Fig. 15 SEM micrograph of oxide particles distribution in the 2S3 alloy; 830 oC/20 h/air; 50 µm from the surface of the sample.
Fig. 12 Optical microscope micrograph of inte rnally oxidised zone in the 1S6 alloy; 830 oC/20 h/air; fine needle-like oxide pa rticles like pearlite structure are fo rme d because of higher concentration of Mg.
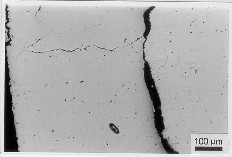

Fig. 13 Optical microscope micrograph of internally oxidised zone and the formation of the main oxide barrier in the 3S2 alloy; 830 oC/20 h/air; passivation has occurred because of too high concentration of Mg
On the basis of our r esear ch it was found out that the size and distr ibution of oxide particles in the selected AgZnMg alloys depend on temper atur e and partial pr essur e of oxygen, as w ell as on the concentration of micr oalloying element. It was also established that the inoculation w eakens by the incr eased temperatur e and par tial pr essur e of oxygen. The most convenient conditions for inoculation occurs at the appropr iate number of nuclei. The corr esponding concentration of micr oalloying element is betw een 0.001 and
0.25 mass% of Mg. On the other side, either too low (0.001
mass%) or to high (0.25 mass%) concentration of oxide nuclei causes the formation of lar ger oxide particles (polyhedr ons, needles and lamellas). Too lar ge concentr ation of microalloying element leads dir ectly to passivation.
The mechanism for the successful nucleation in modified silver based alloys is based on the sufficient number of nuclei of oxide particles of micr oalloying element which ar e uniformly disper sed in metal matrix and cause the pr olongation of ZnO pr ecipitation.
[1] Wag ne r, C.: Z. Ele ktroc he m, 63(1959), 772.
[2] Böhm, G.; Kahlwait, M.: Ac ta me tall., 12(1964), 641 -648.
[3] Hauffe , K.: Re aktio ne n in und an fe ste n S to ffe n, S pringe r Ve rlag,
Be rlin(1955).
[4] S töc ke l, D.: Kine tik de r inne re n Oxy datio n vo n Silbe r-Aluminium- Leg ie runge n, Me tall, 7(1971), 755 -760.
[5] Rapp, R.A.: Ac ta me tall., 9(1961), 730 -441.
IJSER © 2012 http:// www.ijser.org
Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h, Vo lume 3, Issue 1, January -2012 7
ISS N 2229-5518
[6] Bezjak, J.: Ph. D. Disse rtatio n, Unive rsity o f Ljubljana, S love nia(1995).
[7] Bezjak, J.: B.Sc. wo rk, Unive rsity o f Ljubljana, S love nia(1986) [8] Bezjak, J.; Ko sec, L.: Z. Me tallkd. 90(1999), 159.
[9] R.A Rapp, D.F Frank, J.V Armitag e Acta Meta llurgica , Volume 12,
Issue 5, May 1964, Pages 505-513
[10] Nic ho las Winog rad, W. E. Baiting e r, J. W. Amy and J. A.
MunarinVo l. 184 no . 4136 pp. 565 -567
DOI: 10.1126/sc ie nce .184.4136.565
[11] V.A v an Rooije n†, E.W v an Roye n† , J V rije n‡ , S Rade laar† , 2003.
[12] C.P. Wu, D.Q. Yi, J. Li, L.R. Xiao , B. Wang, F. Zhe ng. Jo urnal o f Alloy s and Co mpo unds, Vo lume 457, Issue s 1-2, 12 June 2008, Pages 565-570.
[13] Juan F. Garc ía Martín, Se bastián S ánc he z and Re naud Me tz , 2011.
Kine tics Mo de l o f the The rmal Oxidatio n o f Indium Powde r.
[14] G. Ko ro tce nkov, The ro le o f mo rpho logy and c ry stallog raphic
struc ture o f me tal o xide s in re spo nse o f co nduc to me tric - ty pe g as se nso rs, Mate r. Sci. Eng . R 61 (2008) 1 -39.
[15] E. Co mini, C. Baratto , G. Fag lia, M. Fe rro ni, A. Vo mie ro, G.
S be rveg lie ri, Quasi-o ne dime nsio nal me tal o xide se mico nduc to r:
pre paratio n, c harac te rizatio n and appl ic atio n as c he mic al se nso rs,
Prog . Mate r. Sc i., (In Press), Av ailable o nline 10 July 2008.
[16] A. Kolmakov , X. Che n, M. Mo scov its, Func tio nalizing nanowires
with c ataly tic nano partic les fo r g as se nsing applic atio ns, J. Nano sc i. Nano tec h. 8 (2008) 111 -121.
[17] M. Tie mann, Po ro us Me tal Oxide s as Gas Se nso rs, Che m. Eur. J.
13 ( 2007) 30, 8377-8388
[18] G. Ko ro tce nkov, Me tal o xide s fo r so lid state g as se nso rs. What
de te rmine s o ur