
Figure 2: Graph showing percentage growth of H37Rv, TMC-237 and 28-25271 strains of MTBagainst concentration in μg per ml of medium of rifampicin and the ethanol extract of TinosporaCordifolia

International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2953
ISSN 2229-5518
The anti-mycobacterial activity of Tinospora Cordifolia medicinal plant used for the treatment of leprosy and Tuberculosis
ABSRTACT:
Tuberculosis, MTB, is a common, and in many cases lethal, infectious disease caused by various strains of mycobacteria, usually Mycobacterium tuberculosis. Tuberculosis usually attacks the lungs but can also affect other parts of the body. It is spread through the air when people who have an active MTB infection cough, sneeze, or otherwise transmit their saliva through the air. Most infections in humans result in an asymptomatic, latent infection, and about one in ten latent infections eventually progress to active disease, which, if left untreated, kills more than 50% of those infected. Many communities in south India use medicinal plants to treat various infections, there are claims that some medicinal plants can treat Tuberculosis. Chloroform and Ethanol extracts of Tinospora Cordifolia collected from south India were screened against three strains of mycobacterium tuberculosis using agarwell diffusion method.H37Rv rifampicin resistant TMC-237 and a non resistant wild strain (21102011-DTB-NL).MIC and MIB were determined using agar dilution method on middle brook 7H10.the ethanol extract showed the highest activity against three strains used ,withzone of inhibition of 19.5-23.0 mm and MIC values of 20
Micro g/ml for H37Rv and 15 Microg/ml for both TMC-237 and wild strain .the values for rifampicin were 1.0 Micro g/ml for both H37Rv and wild strain but rifampicin showed activity on TMC-237.THE MBC values for the ethanol extract of Tinospora Cordifolia was 25Micro g/ml for the H37Rv, and 20Micro g/ml for both TMC-227 and wild strain of MT .the MBC rifampicin was 2.0Micro g/ml for both H37Rv and the wild strain. As per Result conclude that Tinospora Cordifolia contains principles active against M. Tuberculosis.
INTRODUCTION:
Tuberculosis remains a devastating health problem in third world countries .Globally more than one third of the world population approximately 2 billions are infected with the bacterium that causes TB(1,2)Tuberculosis is acommon infectious disease caused by various strains of mycobacterium tuberculosis(3)TB attacks the lungs but can also affect other parts of the body.it is spread through the air when people who have an active MTB infection cough,sneeze,otherwise transmit through the air.One third of world population is thought to have been infected with MTB (5,6) and new infections occur at arate of about one per second (5) in 2007 there were an estimated 13.7 millions chronic active cases (7) and in 2010 approximately 8.8 millions new cases ,14.5 million deaths ,mostly in developing countries were estimated (8)the absolute number of TB cases has been
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2954
ISSN 2229-5518
decreasing since 2006 and new cases since 2002,in addition more people in the developing world and their immune system are more likely to be compromised due to higher rates of AIDS (9)Dstribution of TB is not uniform across the globe,about 85% of the population in many asian and African inhabitants test positive in TB test.,while only 5-10% of the US population test positive(10)The emergence of multi drug resistance strains of MTB and more recently extensively drug drug resistance TB poses a formidable challenge to the control of the disease(2)there is aneed to develop new drugs for the control of TB , particularly towards the multi drug resistance and extensively drug resistant tuberculosis strains.there is also need to alleviate the shortcoming of current drug regims by developing safer ,more effective and more affordable agents that can act over short periods of time. Medicinal plants have been a source of effective chemotherapeutic agents for various infectious diseases and there is growing interest in the development of therapeutic drugs of plant origin. Many communities and tribes of India in general, and south India in particular , traditionally use medicinal plants the treat various infections
.TinosporaCordifolia is one of the plant claimed to treat TB and is widely used in some parts of south INDIA (11).. TinosporaCordifolia is a dioecious climbing, woody shrubs very common in all places in forests and among bushes. and belongs to the family of menispermaceae. It is highly invasive and currently occupies a large percentage of the vegetation .
Various uses of TinosporaCordifolia have been reported .It is mainly used as herbal medicine , it is use as a source of microbicides , fungicides and nematicides, insecticides have also been reported (12) In traditional use in south India the leaves of the plant chewed with rock salt and the extract swallowed to get cure from gout, whole plant can be useful for polar , digestive problems and diarrhoea. Chemical compounds isolated from extracts of leaves, stem of TinosporaCordifolia are reported to have shown to exhibit antimicrobial, fungicidal, insecticidal and nematicidal activity. (12) There are also reports that TinosporaCordifolia compound isolated from the extract can be applied as a weed killer and has been tested on the water hyacinth with some success. it is further reported that verbocoside , a compound isolated from Tinospora cordifolia extracts has been demonstrated to posses ant microbial immunosuppressive and antitumor activities. Use of T. cordifolia oil in treatment of skin itches and an antiseptic for wounds and externally for leprosy and scabies also reported (12) Use of TinosporaCordifolia extracts in folk medicine for treatment of leprosy , emetic, dyspepsia, anemia, jaundice, diabetes, eczema, fever, high blood pressure, urinary disorders, catarrhal infections, tetanus, rheumatism, malaria , is also reported. In this study we investigated the antimycobacterial activity of stem extracts from Tinospora Cordifolia.
METERIALSANDMETHODS:
Stem parts of TinosporaCordifolia collected from Tirumala forest area approximately at
(Latitude and longitude .) The team included a Taxonomist, Local healer and investigator.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2955
ISSN 2229-5518
The plant collection was done during the rainy season .At root, leaves , shoot and flowers were used for identification. Stem parts of TinosporaCordifolia were shade dried at room temperature The dried stem parts Were weighed and shaded dried at room temperature. The powdered plant material was percolated regularly for five days with 500 ml of chloroform , ethanol and water respectively. Filtration was done and dry extract collected by rotary evaporator at 400 c and then dried at room temperature. Chloroform , ethanol and water extracts were weighed and yield determined. These dry extracts were stored at room temperature for further analysis. STRAINSANDCULTUREPREPARATIONOFTMC:
Strains of M. Tuberculosis were obtained from the Regional Clinical Research Centre (RCRC), Hyderabad, India. Three strains used in the study .They include the rifampicin resistant (21102011-DTB-NL), rifampicin resistant (TMC-237 ) strain and the H37Rv strain. H37Rv and TMC-237 are used as standard MTB strains. 21102011-DTB-NL MTB strain was isolated from an Andhra Pradesh patient by RCRC. Stored Strains were cultured using Middle brook 7H10 agar supplemented with oleic acid albumin catalase. the work was done in utmost sterilized conditions with safety cabinet using Pereira et al (13) positive cultures were confirmed using fluorescence microscopy with Rhodamine – auramine stain (14,15)
ANTIMYCOBACTERIALSENSITIVITYTESTING : Disc diffusion and agarwell dilution method was used for determination of anti
mycobacterial activity.Dried plant extract of ethanol and chloroform crude dissolved in
DMSO to a concentration of 50 mg/ml to make astock solution .Stock colution was sterilized using 0.2 Micro meter sterile acrodic filter membrane .water extracts were
dissolved in sterile distilled water .50 micrograms per well was used for the general susceptibility test.
A stock solution of rifampicin 3.0 mg/ml was prepared from stock solution of 5 ml.A concentration of 1.0 mg/ml was used general susceptibility test for rifampicin .The medium for incubation was performed in sterile middle brook 7H10 agar in 90 mm in diameter Petri dishes with quadrants.In each quadrant of the petriplate was filled 10.ml of the medium. The medium I the quadrant was inoculated using spreading method so that a uniform surface distribution of innoculum was obtained. Wells of plate 5.0 mm diameter and 2.5 mm depth were bored within standard method. 50 micro litres of test extract was loaded in the first quadrant and concentration of the extracts 50.0 micro grams per well.
A volume of 50 microlitre of the 1.0 mg/ml solution rifampicin and equal volumes of the plant extracts were loaded to the well of the second quadrants .the well of the third quadrant was left as control,fourt quadrant was filled with solvent as control.the Petri plates were left in the laminar air flow overnight to allow diffusion of the extract and drug
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2956
ISSN 2229-5518
and incubated at 370 C in a carbon dioxide incubator for up to 25-28 days.Activity of plant extract and rifampicin drug determined from the zone of inhibition surrounding the well.The sensitivity of MTB to the extract and drug was determined by measuring zone of inhibition using amillimeter zone scale. Three replicates of each test were performed for three strains of MTB. A MIC was determined for the ethanol extract by distributing serial dilution method of test extract. A concentration of 5.0 to 50.0 mg/ml McFarland No1 standards were used in the inocculum preparation. After incubation , according to the lowest concentration that inhibited growth of the bacteria in the medium was taken to be an estimate of the MIC of the plant extracts and the drug
DETERMIATION OF MIC AND MIB
TinosporaCordifolia. Ethanol extract concentrations were introduced into the medium at a concentration of 5.0 mg/ml to determine MIC. It was taken the concentration of the
rifampicin or plant extracts that inhibit growth of MTB by more than 99% , and also in comparison to the positive growth control. The refereed study conducted in fresh cultures of MTB based on McFarland No1 standard (16)
MIC values were defined by the agar dilution method, serial dilutions of the drug and plant extract were made and each sample sterilized and cooled upto 45 to 500 C . the concentrations were made at 15 ml /ml of medium .the petriplates were filed with 10ml per quadrant .The growth control was maintained in one quadrant of a petriplate with no drug
.These petriplates were inoculated with 10-2 dilution of inoculum at McFarland standard
No.1.activity of the sample determined from the number of colonies in all quadrants.
The cconcentration ranges 20mg/ml to 40mg/ml were used to estimate MIC .after the period of incubation number of colonies on the rifampicin contained quadrant Petri plates were determined and expressed as a percentage s of those on the drug free quadrant Petri plates .it was concluded that concentration of the drug or plant extract that prevented growth more than 99%.Verified with untreated controls .Inoculum for the MBC test collected from the medium in the rifampicin inhibited quadrants in the MIC test were scraped and 10 times dilution on inoculum were prepared using the usual procedure of preparing inoculum Fresh Brook7H10 in 90 mm plates . this inocuation procedure for incubation was repeated for up to eight weeks .
Toxicity test on the crude ethanol extract of the active extract of TinosporaCordifolia was done on mice, Mus musculus the test was carried out as described by josh 1984 using mice of both sexes , for each concentration (17). The mice were fasted overnight and different concentrations of doses were used .A pair of mice using widely separated doses at
10,25,50,100 and 500mg/kg/body weight by oral route using a syringe fitted canola. And
some acute drug effects were also studied in 24 hours.
RESULT:
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2957
ISSN 2229-5518
There was significant diffusion of well increased due to plant crude extracts concentration.drug does not shown activity pn TMC-237 at 1 micro grams /mlof medium.in the case of 2825271 and H37Rv strains showed complete clearance of the quadrants.Plant water extracts showed no activity at the concentrations used,qnd not treated further.the ethanol ,chloroform extracts of T.Cordifolia showed significant activity against MTB strains,and further treated at high concentration from 5 to 50 micrograms/ml.the activity of drug was ten folds for active plant extracts .for all the MTB strains no sign of inhibitory activity .H37Rv and wild strains were completely cleared by Rifampicin. The ethanol extract of TinosporaCordifolia stem parts showed the highest activity but it wasLess active than drug on H37Rv and wild strains.the most significicant activity was with the chloroform extracts of T.Cordifolia showed activity but it was less activity than all the three strains of MTB and was less active than its ethanol extract counter part (fig1)The ethanol extract of TinosporaCordifolia which showed an MIC value of 20 æg/ml for H37Rv and15 æg/ml of medium for TMC-237and
The wild strain , compared to drug which shows MIC values of 1.5 Micrograms /ml of medium .In the case of H37Rv and wild strain it was ineffective against the drug resistant strain TMS-237 .The drug completely showed inhibition of growth for wild strain and H37Rv at 2.0 micrograms /ml of medium but was unable to inhibit TMS-237 strain at 3.0 micrograms /ml of medium .This showed an exact contrast with ethanol extract of T. Cordifolia which was active against all the strains MTB used. When pairs of mice were given oral doses, in a referral study using the ethanol extract of TinosporaCordifolia
The lethal doses for animals found to be more than 500 mg/kg body weight. This was beyond the range considered acute toxicity. In this acute toxic conditions mice sedated for six hours and there was no anaesthetic effects a he animals still respond to a pinch on a tail.But there was a little bit lack of breathing and insomnia at doses of 250 mg/Kg body weight and above. However the animal showed normal activity after 24 hour observation
(Table 1,2)
DISCUSSION
The ethanol stem part extract of TinosporaCordifolia showed best activity among the three extracts against three bacterial strains of MTB H37Rv, TMC237, and wild strain. Rifampicin shown more active than ethanol extract against the two strains. Ethanolic extract of TinosporaCordifolia showed activity against drug resistant strain. The stem part extracts of T. Cordifolia was found non toxic to animals. and these studies reported more interest in the medicinal properties of plants.Comparatively low activity of the extracts of TinosporaCordifolia and other extracts compared to rifampicin is one of the most powerful among the anti TB drugs. Pure compounds may be more potent , however T. Cordifolia stem extracts showed an advantage than drug by being highly active against then drug resistant strain. Further studies needed to conclude this. This study reveals
possibility of obtaining versatile compounds for treatment of TB. Therefore , need to carry some more studies in animal models of TB to obtain more reliable findings
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2958
ISSN 2229-5518
Figure 1: Graph of diameters of zone of inhibition against the crude extracts of chloroform and ethanol from the medicinal plants used at 50.0 μg per well, using the agar well diffusion method.

Figure 2: Graph showing percentage growth of H37Rv, TMC-237 and 28-25271 strains of MTBagainst concentration in μg per ml of medium of rifampicin and the ethanol extract of TinosporaCordifolia
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2959
ISSN 2229-5518
Table1:The activity against H37Rv,TMC-237,and 21102011-DTB-NL strains of MTB
strains of varying concentrations of the Ethanol extract of T.cardifolia.
S.No | Concentration (mg/ml of medium) | Number of colonies | ||
1. | Control | H37Rv | TMC-237 | 21102011-DTB-NL |
2. | 10.0 | Not Done | Not Done | Not Done |
3. | 15.0 | Not Done | 20+2 | 18+3 |
4. | 20.0 | Not Done | No growth | No growth |
5. | 25.0 | 18+5 | No growth | No growth |
6. | 30.0 | No growth | No growth | No growth |
7. | 35.0 | No growth | No growth | No growth |
8. | 40.0 | No growth | No growth | No growth |
Table2:The activity of H37Rv,21102011-DTB-NL strains of MTB of different concentrations of Rifampicin drug.
S.No | Concentration (mg/ml of medium | Number of colonies | |
1. | Control | H37Rv | 21102011-DTB-NL |
2. | 1.0 | Not done | Not done |
3. | 1.5 | 10+5 | 8+3 |
4. | 2.0 | No growth | No growth |
5. | 2.5 | No growth | No growth |
6. | 3.0 | No growth | No growth |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2960
ISSN 2229-5518
Figure 3: Graph showing percentage growth of H37Rv,TMC-237,and 21102011-DTB-NL
strains of MTB against micrograms per ml of concentration of Rifampicin of T. Cardifolia.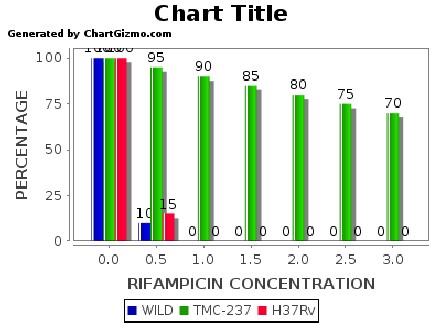
Figure 4: Graph showing percentage growth of H37Rv,TMC-237,and 21102011-DTB-NL strains of MTB against micrograms per ml of concentration of Ethanol extract of T. Cardifolia.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2961
ISSN 2229-5518
REFERENCES
1. World Health organization (2006). Global TuberculosisDatabase. [Online Tuberculosis Database as of
21st March2005].
2. Centres for Disease Control (2005). Worldwide emergenceof Mycobacterium tuberculosis with extensive resistance to
second-line drugs. Trends in Tuberculosis – United States, MMWR;55(10):
3. Kumar V, Abbas AK, Fausto N, Mitchell RN (2007). Robbins Basic Pathology (8th ed.). Saunders
Elsevier. pp. 516–522. ISBN 978-1-4160-2973-1.
4. Konstantinos A (2010). "Testing for tuberculosis". Australian Prescriber 33 (1): 12–18.
5."Tuberculosis Fact sheet N°104". World Health Organization. November 2010. Retrieved 26 July 2011.
6. Jasmer RM, Nahid P, Hopewell PC (2002). "Clinical practice. Latent tuberculosis infection". N. Engl. J. Med. 347 (23): 1860–6. doi:10.1056/NEJMcp021045. PMID 12466511., which cites Dolin PJ, Raviglione MC, Kochi A (1994). "Global tuberculosis incidence and mortality during 1990-2000". Bull World Health Organ 72 (2): 213–20. PMC 2486541. PMID 8205640.
7.World Health Organization (2009). "Epidemio lo gy". Global tuberculosis control: epidemiology, strategy, financing. pp. 6–33. ISBN 9789241563802. Retrieved 12 November 2009.
8"The sixteenth global report on tuberculosis". 2011.
9. Lawn, SD; Zumla, AI (2011-07-02). "Tuberculosis". Lancet 378 (9785): 57–72. doi:10.1016/S0140-
6736(10)62173-3. PMID 21420161.
10.Kumar V, Abbas AK, Fausto N, Mitchell RN (2007). Robbins Basic Pathology (8th ed.). Saunders
Elsevier. pp. 516–522. ISBN 978-1-4160-2973-1.
11.Asthana JG, Jain S, Mishra A, Vijaykanth MS. Evaluation of antileprotic herbal drug combinations and their combination with Dapsone. Indian Drugs 2001;38:82-6.
12.S.S. SINGH, S.C. PANDEY, S. SRIVASTAVA, V.S. GUPTA, B. PATRO, A.C. GHOSH Indian Journal of
13. Pereira M, Tripathy S, Inamdar V, Ramesh K, Bhavsar M,Date M et al. (2005). Drug resistance patterns of Mycobacterium tuberculosis in seropositive and seronegative HIV-TB patients in Pune, India. Indian J Med Res, 121 (4): 235-239.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2962
ISSN 2229-5518
14. McCarter YS and Robinson R. (1994). Detection of acidfast bacilli in concentrated primary specimen smears stained with rhodamine –auramine at room temperature and at 37oC. Journal of Clinical Microbiology. 32 (10):
15. Kent BD, Kubica GP (1985). Public health mycobacteriology guide for level III Laboratory. Atlanta.
16.N. MARTIN-CASABONA,1* D. XAIRO´ MIMO´ ,1 T. GONZA´LEZ,1J. ROSSELLO´ ,2 AND L. ARCALIS Received 9 January 1997/Returned for modification 11 June 1997/Accepted 14 July 1997
Copyright © 1997, American Society for Microbiology
17. Lee, C.N., Heifets, L.B. (1987). Determination of minimum inhibitory concentration of anti- tuberculous drugs by radiometric and conventional methods. Am Rev Respir Dis 136 (2): 349-352.
IJSER © 2013 http://www.ijser.org