International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1278
ISSN 2229-5518
The Study of Controlled Relaese of Indomethacin
Drug From Prepared Apatite Compounds
Containing The Elemantal Calcium And Strontium
Roua Hussein Nasser, Abdelameer H.Taobi and Zaki N. Kadhim
Abstract— Apatite compounds has wide applications in the medical and non-medical field and are very stable compounds .Different compounds of apatite were prepared and identified such as a that of calcium hydroxyapatite, Strontium Fluorapatite compound and Strontium –Calcium hydroxyapatite compound . All these compounds were identified by Infrared spectroscopy and X-rays, which confirmed the presence of the prepared compounds. The release of a indomethacin drug in a laboratory in the prepared compounds were studied. Porosity tablets of the compounds were prepared using the material (Micro Crystalline Cellulose) as a porous. then removed the used cellulose material as a porous by heating in oven at 600 C° for two hours. These tablets was examined using scanning electronic microscope (SEM) which shows the presence of different porous sizes in the prepared tablets .
keywords — Apatite ,drug delivery , hydroxyapatite , flourapatite , x-ray , micro crystalline cellulose , SEM, .
—————————— ——————————
1 INTRODUCTION
hey mineral apatite in bones , teeth , sedimentary rocks , igneous and metamorphic rocks. They have many colors such as :( green, blue, yellow, white, red, pink, brown and
colorless (1) .The apatite compounds are thermally very stable which attract the attention of many scientists and researchers due to its composition which has the ability to resist high temperatures (2,3) .
Hydroxyapatite (HAP) is the inorganic compound of calci- um phosphate which is made of ceramic materials, biological compounds
Group, and is a metal component President in bones , teeth
,rocks and in the sea coral (4,5) . Hydroxyapatite is a crystalline
substance which is organized hexagonal crystals binary sys-
tem engineering pyramid .The molecular formula is: Ca10
(PO4) 6 (OH) 2 .
The term apatite can be described in general formula: M10
(XO4) 6Z2 , as M represents a metal which may be (Ca2 +, Ba2
+, Sr2 +, Pb2 +, Mg2 +, Zn2 +, Si2 + or Na +), and X represents
the (P5 +, V5 + or Mn5 + ), while Z represents the ions (Cl-, F-
or OH-) (6) .
Hydroxyapatite attract the attention of researchers in the
field of biomedical applications due to its great similarity in the composition and structure between teeth and bones. It has the ability to absorb some of the organic compounds and therefore has been used widely as agents of separation of pro-
teins and amino acids in resins Chromatography column. Also used as additives in toothpaste, and in the gas sensor compo- nents in ionic conductors, and medically as Osteoimplants (7,8) .
Hydroxyapatite active ingredient Bioactive biology and material of harmony vital and biological Biocompatibility so coated by some of the parts that are grown in the body, such as parts made of titanium and stainless steel , Other character- istics of hydroxyapatite which makes material have a vital agree that it does not dissolve in the body of the object neigh- borhood due to very high melting point "up to 1250° "
In recent years several types of composite materials (poly- mer-hydroxyapatite) as materials to replace the bone are de-
veloped. The purpose of the integration of the polymer with HAP is to support and improve the properties of bonding with the bone. It has been found that the HAP in such com- pound have around from the polymer material is biologically active "compound linked to the bone with the same time and can improve the mechanical properties, especially the flexibil- ity and hardness coefficient Elastic Modulus and Hardness (9,10)
, The HAP's complex structure has advantage of adding a good absorbance of various pharmaceutical products such as antibiotics, hormones and steroids. Used as a composite HAP with antibiotics HAP-Antibiotics and successfully used for the launch of slow pharmaceutical compounds (11-13), has proved of medication received good efficiency in the treatment of dis- eases such as system, Bone infections Osteomyelitis and oste- oporosis (osteoporosis) Osteoporosis and bone cancer Osseous Cancer (14) .
The aim of these paper to develop some composite of HAP
with indomethacin as slow release device . .
1.1 EXPERIMENTAL
A) Preparation of Strontium Flourapatite
A solution 100 ml containing ( 4.356 g) of di ammonium
hydrogen phosphate and (1.152 g) of ammonium fluoride are
mixed and heated to the boiling point at (pH = 9) by adding
ammonium hydroxide solution and then added strontium
nitrate solution (8.295 g) slowly to the hot solution with vigor-
ously stirring for 3 hours and then filtered by Vacuum Filtra-
tion and wash with distilled water. The sediment placed in the drying desiccator at room temperature for 12 hours before grinding to a powder. The powder heated at a temperature of
150 C ° for two hours and then at a temperature of calcined at
1000 C ° for two hours (15,16) .
B) Preparation of Calcium-Strontium Hydroxyapatite
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1279
ISSN 2229-5518
A solution 500ml contain (0.25 mol) of calcium nitrate tetra hydrate and (0.25 mol) of strontium chloride hydrate , and the solution 500 ml of Di ammonium hydrogen phosphate (0.3 mol ), are placed in flask and heated to 80 C ° for 3 h at (pH =
10 ) by adding ammonium hydroxide solution, Then the solu- tion washed several times with distilled water and then rinse with ethanol, dried filtrate at 100 C ° for one hour one and placed at 1000 C ° for two hours (17) .
C) Preparation of Hydroxyapatite Powder Using Sol-Gel
Compound
Phosphoric acid solution (0.25 M) (pH = 10) was added with
stirring to calcium nitrate tetra hydrate solution (1 M) very
slowly while maintaining (pH = 10) by adding ammonium
hydroxide solution. The solution is shaken vigorously for one
hour and left at room temperature for 24 hours, then dried at
65 C ° for 24 hours and washed with water several times to
get rid of nitrate and ammonium ions and heated to 1000 C °
for two hours (18) .
D) Preparation of porous tablets of prepared compounds
Powder material (Microcrystalline cellulose) was mixed
with prepared compounds by ratio (25% w / w) to get the
porous. Samples compressed tablets on the weight of each
tablet form (0.5 gm), then these tablets are exposed to temper-
ature 600 C ° for two hours to get rid of cellulose material and
get a clear picture of porous in the scanning electronic micro-
scope device.
1.2 Drug Loading and Release
(0.5 gm) of the drug was used with (0.5 g) of the tablets pre- pared compounds in (20 ml) of buffer solution at (pH = 7.4) for
24 hours with stirring (shaker).The tablet placed in (10 ml) of buffer solution at (pH = 7.4) and measured absorbance at different times to follow up the release at a wavelength of 320 nm, The absorbance measured at time per 15 minutes in the first two hours, then measurement every 30 minutes in the next five hours, and every four hours for the remaining in 24 hours.
2
2 RESULTS AND DISCUSSIONS
2.1 IDENTIFICATION OF COMPOUNDS BY IR
SPECTROSCOPY
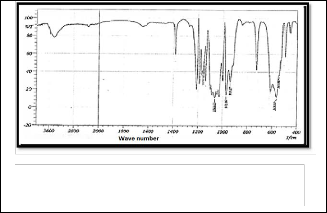
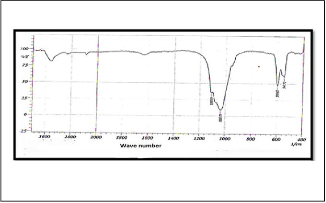
is shown in Figure (1) . The peak appeared at (3440 cm-1), which is back to the group (OH Stretching), and the peak at (1064.63 cm-1) which is back to the group (PO Asymmetric Stretching), while the peak back to the group (PO Symmetric Stretching) is appeared at (1002.92 cm-1), and the peak to (PO Deformation Group) was appeared at (563.18 cm-1) . Figure (2) represent an infrared spectrum of Strontium Flourapatite compound (SrFA), the band belong to (PO Asymmetric Stretching group) appeared at (1078.13 cm-1), while the band belong to (PO Symmetric Stretching Group) is appeared at (1035.77 cm-1) , also the one peak at (592.11 cm -1) is return to (PO Deformation group). The Infrared spectrum of Strontium Calcium hydroxyapatite compound (SrCaHA) is shown in Figure (3). The peak at (3480cm-1 ) due to the (OH ) group , while the band due to (PO Stretching Symmetric Group) was appeared at (1035.70 cm -1) , and the (PO Stretching Asymmet- ric group ) appeared peak at (1108.99 cm-1 ) also appeared peak back to the (PO Deformation) group at (599.82 cm-1). Ta- ble 1 show the main peak in the prepared
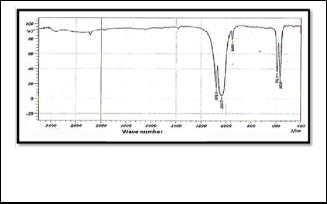
Fig. 1 Infrared spectrum of calcium hydroxyapatite pre- pared by Sol-Gel
Fig. 2 infrared spectrum of Strontium Flourapatite com- pound (SrFA)
————————————————
• Author name is currently pursuing masters degree program in electric power engineering in University, Country, PH-01123456789. E-mail: au- thor_name@mail.com
• Co-Author name is currently pursuing masters degree program in electric power engineering in University, Country, PH-01123456789. E-mail: au- thor_name@mail.com
(This information is optional; change it according to your need.)
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1280
ISSN 2229-5518
Fig. 3 infrared spectrum of Strontium Calcium hydroxyap- atite compound (SrCaHA)
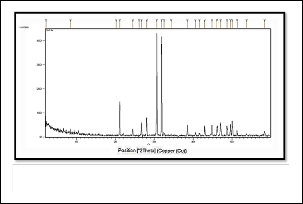
Fig. 5 x-ray diffraction spectrum of SrFA
2.2 IDENTIFICATION OF COMPOUNDS BY X-RAY DIFFRACTION
(XRD)
The X-ray diffraction (XRD) of th
e prepared compounds are shown in figs (4-6) which repre-
sents Finger Print for compounds . The most important thing
to confirm the installation of the compound is the value of d
value , which represents the distance between atomic planes
in the crystal (19) . The value of d2ᶿ was calculated according to
the equation derived from Barak Bragg Law (20).
d (nm) = 154/2 sin Rᶿ
As RᶿR represents the angle between the incident X-ray beam
and the levels of the crystal surface.
It showed a very high correspondence with d2ᶿ calculated for
apatite compounds and installed the values in the literature
(XRD) approved (21) .
Fig. 6 x-ray diffraction spectrum of CaSrHA
3 QUANTITATIVE ANALYSIS
Both calcium and phosphate represent the major inorganic components in bones and teeth and hydroxyapatite. Spectral complex of Phosphomolybedenum blue method was used to determine the phosphate concentration in the prepared com- pounds, at a wavelength of 660 nm (20). The results were shown in Table (5).
Flame atomic absorption spectrometry was used to meas- ure the elemental concentration of calcium and strontium in the prepared compounds and the results were shown in Table (1)
TABLE 1
the ratio f calcium and strontium in the prepared compounds


Contraction of Percentage of Percentage of compounds phosphate calcium strontium

(ppm) %W/W %W/W
. Fig.4 x-ray diffraction spectrum calcium hydroxy- apatite prepared by Sol-Gel
Calcium Strontium 0.830 18.39 21.72
Hydroxyapatite
(CaSrHA)
Strontium 2.250 ــــــــــــــ 32.27
Flourapatite (SrFA)
Calcium 0.867 27.086 ـــــــــــــ
Hydroxyapatite powder
(CaHAP Powder)
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1281
ISSN 2229-5518
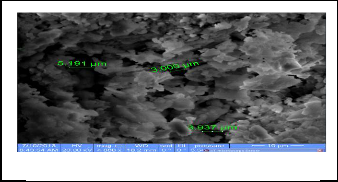
5 Scanning Electron Microscope
The Scanning Electron Microscope (SEM) gives pictures of the samples by scanning the beam center of electrons with high energy to generate a variety of signals on the surface of solid samples signals that arise from interactions electronic form giving information about the external morphology and chemi- cal composition and crystal structure of the samples , as can the number of times magnification control device (SEM) to about 500,000 visits (22) . The following SEM figures show a picture of the prepared compounds . It is shown the porous in prepared tablets and size of these porous . the figures ( 7-9) represent images prepared compounds in scanning electron microscope
.
Fig.7 CaHAP powder
patients who suffer from arthritis treatment . Prepared porous tablets by mixing Micro Crystalline cellulose material with the prepared compounds by 25%W/W) and through curves were obtained shown that the release mechanism of the drug is by diffusion process, The measured absorbance with time at wavelength 320 nm , show that the release of the drug ratio varies from compound to another.
The release of Strontium flourapatite compound (SrFA), who was the loading percentage (42.50%), which reached release ratio (22.50%) two hours later, after three hours later was (35.46%), , And reached the level at (66.58% ) which it stabi- lized After 8 hours and remained stable at this level even after
24 hours. The compound Strontium Calcium Hydroxyapatite was loading (47.8%), it is found that the release reached (24.63%) after half an hour, after one hour (56.04%) , after two hours and the release percentage was (82.09%) ,and reached the level at (82.44% ) which it stabilized After 5 hours and remained stable at this level even after 24 hours. The release in Calcium Hydroxyapatite compound prepared by sol-gel, which was loading (32.8%), the release reached (19.89%) after an hour, two hours after the release reached to (44.35%), after
4 hours reached (78.23%) and reached the level at (78.54%) which it stabilized After 6 hours and remained stable at this level even after 24 hours. (Fig. 10) Shows the release of the drug from prepared compounds.
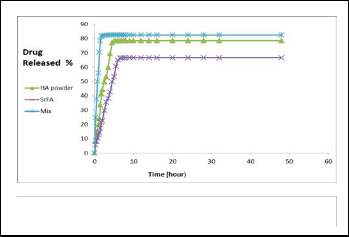
Fig. 8 SrFA
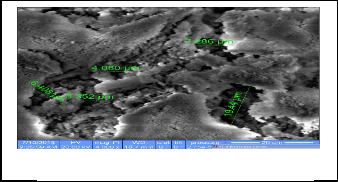
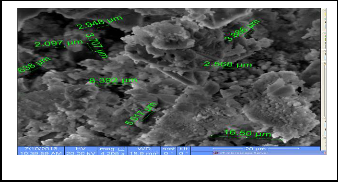
Fig. 10 the release of the drug from prepared compounds.
Fig. 9 SrCaHA (mix)
6. The Loading and Release of Drug
The loading and release of the drug was studied under phys- iological conditions (in vitro), as has been loaded prepared compounds tablets with indomethacin drug, which is used in
4 CONCLUSION
Of the study load and release of the indomethacin drug from apatite prepared compounds showing that the release of the drug with the ratio of release take different time depend- ing on the compounds and measure the percentage of calcium and strontium in the prepared compounds.
In future preparation apatite compounds containing other
elements such as barium Ba and silicon Si and Mg magnesium, sodium Na, lead Pb. And replace ions PO4 in CO3
and use of other drugs anti-arthritis is indomethacin .
REFERENCES
[1] Farndon , John. , The practical Encyclopedia of Rocks& Minerals , London: Lorenz Books (2006).
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1282
ISSN 2229-5518
[2] Hidouri M., Bouzouita K., Kooli F., Khattech I., Materials Chemistry and
Physics, 80 , 496 ,(2003).
[3] Sugiyama S., Nakanishi T., Ishimura T., Moriga T.; J. of Solid State Chemistry,
143, 296,( 1999).
[4] Chakraborty S., Bag S., Pal S. and Mukherjee A.K., J. Appl. Crest. , 39, 385, (2006).
[5] Cengiz B., Gokce Y., Yildiz N., et al. , Physiochemical and Engineering As- pects. : 322, 1 , (2008)
[6] Shalaby S.W. and Salz U. , "Polymers for Dental and Orthopedics Applica- tions " Ch.3, P 79, CRC press, Boca Raton , (2007).
[7] Aoki H., Tokyo . , Inc. Ishiyaku. Pub. ,( 1999) .
[8] Kanazawa T. ,Tokyo . Kodansha Scientific Ltd. (1985) .
[9] T. Albrektsson, G. Zarb, P. Worthington, A.S. Ericsson, Int. J. Oral Maxillof.
Impl., 1,11, (1986).
[10] K.P. Andriano, A.U. Daniels, J. Appl. Biomater., 3,197, (1992).
[11] Ginebra MP, Traykova T, Planell JA. J. Control Release.;113(2): 102, (2006).
[12] Schnieders J, Gbureck U, Thull R, Kissel T. , Biomaterials. ;27(23):4239,( 2006). [13] Baro M, Sanchez E, Delgado A, Perera A, Evora C. , J. Control Release. ;
83(3):353, (2002).
[14] Cornell CN, Tyndall D, Waller S, Lane JM, Brause BD. , J. Orthop.
Res.;11(5):619, (1993).
[15] Hidouri M., Bouzouita K., Kooli F., Khattech I., Materials Chemistry and
Physics , 80, 496,( 2003).
[16] Chirantha Prageeth Rodrigo , thesis , University of Colombo, Sri Lanka 2001. [17] Lin . Yingguang , Yang Zhuoru, Cheng Jiang , Wang Lianshi . J. of wuhan
university of Techology-Mater . Sci . (2008).
[18] K P Sanosh , Min-cheot Chu , A Balakrishnan , T N KIM and Seong – JAI Cho. Ball Mater. Sci., Indian Academy of Sciences , no. 5, vol. 32, 465 , ( 2009).
[19] Wien T., Measurement and Acquisition of X-Ray Diffractometry Spectra , Ch.
9, 748, Warsaw ,(1995).
[20] Pardo B., Megademini T. and Ander , J.M., Rev. Phys. Appl. 23, 1579, (1988). [21] Markovie M., Fowler B.O. and Tung M.S., J. Res. Natl. Inst. Technol., 109, 553,
(2004).
[22] McMullan, D. Scanning 17 (3): 175, (2006).
IJSER © 2015 http://www.ijser.org









