International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014
ISSN 2229-5518
1295
The Role of Interferon- Gene Polymorphism in
Spontaneous Abortion
Fatima A. AL-Timimi, Mayyada F. Darweesh, Ali R. Hussein
Kufa university/ Faculty of Science
ABSTRACT
Background: The spontaneous abortion is considered as one of the important clinical problems that may affects pregnant women during the 1st
20-22 weeks of pregnancy causing dangers to the mother and her fetus. In recent years, a lot of studies suggest that recurrent abortion is caused by the genetic factors.
Aim: The aim of this study was to investigate the association of the IFN- +874T/A polymorphisms and level with spontaneous abortion.
Methods: The study group comprised 60 women with spontaneous abortion, and the control group consisted of 30 healthy delivered women. IFN-
Gene polymorphism was detected by ASO-PCR technique and blood level was estimated by ELISA.
Results: The frequency of TT genotype (35%) and T allele (55.83%) was significant higher (P<0.05) in the abortion group than in the control group (13.33%, 35% respectively), also, IFN- level was significantly (P<0.01) higher in abortion group (8±0.4 IU/ml) than in control group (1.3±0.07
IU/ml).
Conclusions: This study concluded that the IFN- +874T/A polymorphisms (TT genotype) and high IFN- level may be associated with spontaneous abortion in Iraqi (Al-Najaf) women.
Keywords: Spontaneous Abortion; IFN; Polymorphism.
—————————— ——————————
Recurrent spontaneous abortion (RSA) is defined as two or more failed pregnancies before the 20th week of gestation (1). Spontaneous abortion is one of the most common complications of pregnancy, with approximately one in every four pregnant women undergoing one or more pregnancy losses (2). Recurrent abortion has been attributed to a large number of etiological factors, in approximately two-third of cases the cause is known to be genetic error, hormonal abnormalities, anatomic abnormalities of the reproductive tract, immunologic factors, infection or systemic disease whereas, one third of all cases is idiopathic (3).
Considerable evidence has accumulated indicating that cytokines play an important role in the maintenance of pregnancy by modulating the immune system. Deregulated immunity has been proposed as a potential mechanism underlying abortion (4). Plasma levels of pro-inflammatory (Th1) cytokines such as IFN- are higher, while levels of anti-inflammatory (Th2) cytokines are lower in women who miscarry than in those who maintain their pregnancy (5).
IFN-γ is produced predominantly by natural killer (NK) cells (in innate immune response), and by CD4 Th1 and CD8 cytotoxic T lymphocyte (CTL) effector T cells (adaptive immunity) (6). IFN- ( as pro-inflammatory cytokine) are considered to be detrimental to pregnancy (7). Trophoblast activated peripheral blood mononuclear leukocytes from women with a history of RSA produce more pro-inflammatory cytokines but less anti-inflammatory cytokines than women without a history of RSA (8).
Many study focused on single nucleotide polymorphisms (SNP) in cytokine genes (9) and various SNPs have been reported to be associated with infectious and inflammatory conditions, including the risk of prelabour rupture of the amniotic membranes and preterm labor (8). Polymorphisms in the promoter regions, exons or introns of certain cytokine genes, influence the level of cytokine production and result in high, intermediate or low levels of cytokines (5). IFN- production is under genetic control of IFN- gene, which is located on the long arm of chromosome 12. It has been shown that different genotype at the site +874 T/A in the first interon of the IFN- gene was associated with the production of IFN-
. Polymorphisms of IFN- genes, influence the level of IFN- production and result in high (TT), intermediate (AT) or low
(AA) levels of cytokines (10).
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014
ISSN 2229-5518
1296
Studies of SNP of IFN- (+874 T/A) in some populations show association with abortion, such in Caucasian Argentine women (11). while in others no association has been observed in Indian women (12). Interestingly, variation in the prevalence of different polymorphic forms is a frequent finding and this makes it necessary to conduct association studies in each individual population. This is the first study in our country, which considered IFN- gene polymorphism in aborted women and compared its production level.
The control group included 30 Iraqi delivered women with no history of abortion (age ranged from 16-44). The patient group comprised 60 Iraqi women with spontaneous abortion (age ranged between 16-45 years). None of all the subjects with any medication would affect the immune system. All subjects were come during the period from 1/9/2013 to
30/11/2013 to Al-Zhra Teaching Hospital, Al-Hakeem Teaching Hospital, and Al-Najaf Private Hospital in Al-Najaf province. From this women, we taken placental biopsies and blood samples.
Genomic DNA was isolated using the Wizard Genomic DNA Purification Kit purchased from Promega (Madison, Wisconsin, USA). The IFN- A874T polymorphism was detected by PCR-ASO using two primer taken from (13):
874 T:
5’TTCTTACAACACAAAATCAAATCT3’
5’TCAACAAAgCTgATACTCCA3’
874 A:
5’TTCTTACAACACAAAATCAAATCA 3’
5’TCAACAAAgCTgATACTCCA3’
The final volume of all polymerase chain reaction (PCR) reactions was 30 ml: 15 μl of 2X Taq PCR Pre-mix (SolGent), 1.5 μl of Common Primer (10 pmol), 1.5 μl of either A allele or T allele Primer (10 pmol), 4 μl of Template DNA and 8 μl of D.D. Water. Reactions were carried by PCR system (Agilent, USA). The cycling conditions were as follows: an initial denaturation at 94°C for 5 min, followed by 35 cycles at 94°C for 30 s, 37°C for 50 s and 72°C for 40 s, with a final extension step of 5 min at 72°C in the last cycle. The amplified products were separated by electrophoresis on a 1.5% agarose gel stained with 3 μl ethidium bromide. The gel was visualized under a UV transilluminator with a 100–base pair ladder (SolGent) and photographed. Two sample products (261 bp) were available for each participant (1 for each specific T or A allele of the IFN-
alleles).
Blood samples were taken from aborted women and controls under aseptic conditions and left for 10 minutes for spontaneous clotting at room temperature before being centrifuged (3000 rpm) to separate the serum. Serum samples were kept frozen at –20°C for determination of IFN- level by ELISA Kit (Biosource).
genotype frequencies of IFN-γ gene were estimated by direct counts and expressed as percentage. Comparison
between patients and controls were done using X2 test. The frequencies of IFN-γ were also tested for Hardy-Weinberg equilibrium by calculating allele frequencies using Pearson’s X2 test with one degree of freedom. Whereas the results of IFN-γ level were expressed as arithmetic mean ± SE. The comparison between the patient and controls were analyzed by Unpaired T-test. P<0.05 was considered statistically significant by using software packages Graphpad prism 6 (Version 6.01) for windows 2007.
The results of SNP analysis of IFN-γ at position (+874T/A) by ASO-PCR, revealed that there are three genotype TT, AT, AA in patients and control group, presented in Table (1). Also, the results showed that IFN-γ +874 TT, AA, AT genotype frequencies were 35%, 23.33%, 41.67% in aborted women and 13.33%, 43.33%, 43.33% in controls, respectively. The frequencies of IFN-γ +874 T, A alleles were 55.83%, 44.17% in aborted women, and 35%, 65% in control, respectively. Distribution of the genotypes in aborted women and controls was consistent with the Hardy-Weinberg equilibrium. The
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014
ISSN 2229-5518
1297
difference in the distribution of IFN-γ genotypes and alleles between controls and aborted women were statically significant (P <0.05), And there was significant prevalence of +874 T allele and TT genotype in aborted women.
Table (1): IFN-γ genotype and allele distributions
Genotypeِ/Allele | Aborted women (60) | Control (30) | ||
Genotypeِ/Allele | No | % | No | % |
TT | 21 | 35 | 4 | 13.33 |
AA | 14 | 23.33 | 13 | 43.33 |
AT | 25 | 41.67 | 13 | 43.33 |
Significant? | P < 0.05, Yes | |||
TT | 21 | 35 | 4 | 13.33 |
AA+AT | 39 | 65 | 26 | 86.67 |
Significant? | P < 0.05, Yes | |||
AA | 14 | 23.33 | 13 | 43.33 |
AA+AT | 46 | 76.67 | 17 | 86.67 |
Significant? | P=0.05, No | |||
T | 67 | 55.83 | 21 | 35 |
A | 53 | 44.17 | 39 | 65 |
Significant? | P < 0.05, Yes |
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014
ISSN 2229-5518
1298

Ladder T A T A T A
500bp
400bp
300bp
261bp
200bp
100bp
1 | 2 | 3 |
TT | TA | AA |
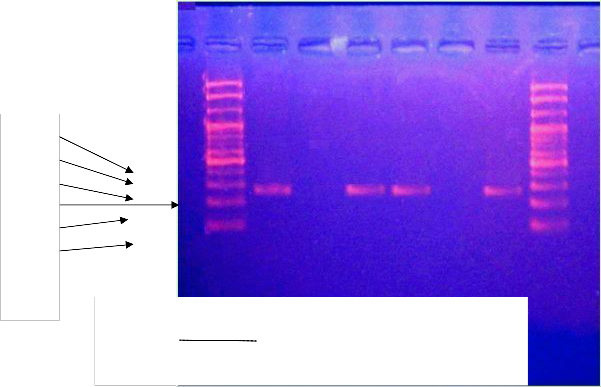
FIGURE (1): ASO-PCR GEL ELECTROPHORESIS OF T/A +874 POLYMORPHISM OF IFN-D GENE. EVERY SAMPLE WAS PRESENTED BY 2
LANES FOR T AND A ALLELES RESPECTIVELY TO AMPLIFY A PRODUCT OF (261BP) OF THREE SAMPLE. LANE 1&2 (SAMPLE 1): HOMOZYGOUS FOR T ALLELE (TT GENOTYPE).
LANE 3&4 (SAMPLE 2): HETEROZYGOUS FOR T (OR A) ALLELE (TA GENOTYPE). LANE 5&6 (SAMPLE 3): HOMOZYGOUS FOR A ALLELE (AA GENOTYPE).
4.4. IFN- LEVELS
The mean serum level of IFN-γ was significantly (P<0.01) higher among cases of aborted women (8±0.4 IU/ml) compared to controls (1.3±0.07 IU/ml) , as shown in Table (2). This elevated level of IFN-γ in aborted women as compare with control is correlated with the significant prevalence of T allele (and TT genotype) in aborted women. In aborted women, IFN-γ TT genotype has significantly (P < 0.05) higher IFN-γ serum production (9.2±0.7) than AA genotype (7±0.6), whereas AT genotype level was intermediate (7.8±0.6) between TT and AA genotype, as shown in Table (3).
TABLE (2): IFN-γ LEVEL.
Parameter | Aborted women | Control | Significant? |
IFN- IU/ml | 8±0.4 | 1.3±0.07 | P < 0.01, Yes |
![]()
TABLE (3): ABORTED WOMEN IFN-γ GENOTYPES AND LEVEL.
Parameter | Aborted women | ||
Parameter | TT | AA | AT |
IFN- IU/ml | 9.2±0.7 | 7±0.6 | 7.8±0.6 |
Significant? | P < 0.05, Yes |
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014
ISSN 2229-5518
5. DISCUSSION
1299
IFN-γ SNP
In the present study, we have made an attempt to study the predisposing effect of SNPs within the IFN-γ gene, which is related to TH-1 cytokine, on abortion among AL-Najaf Iraqi women. This is the first report from this part of the country. This findings demonstrated the susceptible role of the T wild-type IFN-γ in abortion.
The present study suggest that the allele +874 T seems to be a predisposing factor to abortion, in opposition to the role of the allele +874 A, which seems to be a protective factor to abortion. Thus, the production of IFN-γ in individuals homozygous for the (+874) T allele was contributed to their increased risk of developing abortion. In addition, the results seem to reinforce the association of the +874 TT genotype with the susceptibility to the abortion, whereas the +874 AA genotype might be related to protection against abortion or even a partial protection when the allele A is present in heterozygosis (AT).
The results approached with (14) in Indian women, who showed significant association between IFN-γ (+874A/T) SNP with recurrent miscarriage compared with controls, and clarified that T frequency was higher in aborted women than controls. This study also approached with (15), who revealed statistically higher frequencies of IFN-γ genotype TT (+874) in aborted women when compared to general population.
By contrast (16), found that there was no association between IFN-γ +874A/T polymorphisms and the manifestation of RPL, who performed a case – control study in Caucasian women suffering from RPL. This results also disagree with results of (17), who not found any significant associations of RPL with IFN-γ (+874T).
But (18), found that the allele and/or genotype frequencies of the IFN-γ (+874 A/T) polymorphisms significantly different between women with RSA and controls, and explained that this results might be related to ethnic background, environmental factors, selection criteria for studied populations and the possibilities of exist multiple cytokine gene polymorphisms or other genes in linkage disequilibrium that may play a role in RSA.
So the causes of these differences may be explained by the difference in the linkage groups associated with IFN-γ +874 polymorphisms in the geographically different population studied. Hassan et al. (19), showed that SNP allelic and genotypic frequencies for IFN-γ differed significantly between the Caucasian and African American women.
Pravica et al. (20), described a novel single nucleotide polymorphism, T to A, at the 5− end of the CA repeat region in the first intron of the human IFN- γ gene. Sequence analysis for five allele of this microsatellite has shown that allele 1 corresponds to 11 CA repeats, allele 2 (T allele) corresponds to 12 repeats, and alleles 3 (A allele) and 5 have 13–15 repeats, respectively.
IFN- encoded by the IFN- gene and SNP have been reported in first intron (at position +874T/A), thereby directly affecting IFN- production levels (20). The role of IFN- levels in abortion pathogenesis remains controversial. It was suggested that increased IFN- expression was associated with abortion, whereas low levels were linked to successful pregnancy. Thus, IFN- could be an important pro-inflammatory cytokine contributing to the outcome of pregnancy. This study supports the hypothesis that depended on the role of Th-1 produced cytokines in the pathogenesis of miscarriage (21). The results of this study explained that the calculated level of IFN-γ of aborted women was significantly higher than calculated level for controls.
These results approached with (22), who found significantly higher IFN-γ level in RSA. However, a study of cytokine production by peripheral blood cells of women with recurrent miscarriage taken during early pregnancy before miscarriage, has shown opposite effects with increased IL-4 and IL-10 and decreased IFN- (23). Also this study approached with (24), who showed there was high significant elevation of serum IFN-in aborted women compared with normal delivered women.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014
ISSN 2229-5518
1300
IFN-γ was regarded as important indicator for Th1 immune response in this study, because of its Th1 polarizing effect due to its potential role in generating Th1 cells, mediating their effects functions and regulating Thl/Th2 balance (25,26). While other studies have shown that IFN could inhibit human placental trophoblast cell growth and metabolic activity and stimulate apoptosis in vitro (27,28). The pro-apoptotic effects of IFN- γ, in normal, are completely neutralized by epidermal growth factor (EGF) and partially neutralized by basic fibroblast growth factor (FGF-), insulin-like growth factor-1 (IGF-1) and platelet-derived growth factor (PDGF) (29).
This study showed that IFN-γ TT genotype has the highest production capacity followed by AT genotype then AA genotype. This latest finding is similar to that of [10] in Babylon and (30) in Egypt, they confirm this highest production of IFN- γ by TT genetype followed by AT genotype then AA genotype.
The +874 T allele is linked to the 12 CA repeats, whereas the A allele is linked to the 13 CA repeats. The specific sequence of the T allele is found to provide a binding site for the transcription factor (NF-kB). As NF-kB induces IFN-γ expression, this T allele correlates with high IFN- γ expression, whereas the A allele correlates with low expression [31].
Pravica et al. [13], mention that dinucleotide polymorphism was located at the position +874 from the translation start site (+874T/A) which coincides with a putative NF-kappa B binding site which might have functional consequences for the transcription of the human IFN-γ gene, and associated with individual variation in the levels of IFN-γ production. The genotypes at this position, with a low (AA), medium (AT) and high (TT) cytokine production.
Nuclear factor- kappa B (NF-kB) is a protein complex that controls the transcription and production of cytokines (32), and antimicrobial effectors as well as genes that regulate cellular differentiation, survival and proliferation, thereby regulating various aspects of innate and adaptive immune responses. Through a cascade of phosphorylation events, a kinase complex is activated and NF-κB is able to enter the nucleus to upregulate genes involved in T-cell development, maturation, and proliferation (33).
CONCLUSIONS
This study concluded that the IFN- +874T/A polymorphisms (TT genotype) and high IFN- level may be associated with spontaneous abortion in Iraqi (Al-Najaf) women.
7. REFERENCES
1.ASRM - Practice Committee of the American Society for Reproductive Medicine (2012). Evaluation and treatment of RPL - a committee opinion. Fertil. Steril., 98(5):1103-11.
2.Linda, W. and Prine, M.D. (2011). Office management of early pregnancy loss. Am. familiy physican, 84(1):75-82.
3.Cosgriff, T.M.; Martin, B.A. and Fishel, L.A. (2009). Low functional and high antigenic antithrombin III level in a patient with the lupus anticoagulant and recurrent thrombosis. Rheumat. j., 24:94-96.
4.Jenkins C, Roberts J, Wilson R, MacLean MA, Shilito J, Walker J.(2000). Evidence of a th1 type response associated with recurrent miscarriage. Fertil Steril, 73: 1206-1208.
5.McGuire, W.; Hill, A.V.; Allsopp, C.E.; Greenwood, B.M. and Kwiatkowski, D. (1994). Variation in the TNF-alpha promoter region associated with susceptibility to cerebral malaria. Nature,371: 508-510.
6.Schoenborn, JR. and Wilson, CB. (2007). "Regulation of interferon-gamma during innate and adaptive immune responses". Adv. Immunol. 96: 41–101. 42- Liu YJ . "IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors". Annu. Rev. Immunol., 23:275-306.
7.Lissauer, D.; Goodyear, O.; Khanum, R.; Moss, P.A.; Kilby, M.D. (2014). Profile of maternal CD4 T-cell effector function during normal pregnancy and in women with a history of recurrent miscarriage. Clin Sci (Lond). 126(5):347-54.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014
ISSN 2229-5518
1301
8.Bidwell, J.; Keen, L.; Gallagher, G.; Kimberly, R.; McDermott, M.F.; Huizinga, T. et al. (2001).Cytokine gene polymorphism in human disease: On-line databases, supplement 1. Genes Immun, 2: 61-70.
9.Raghupathy, R.; Makhseed, M.; Azizieh, F.; Omu, A.; Gupta ,M.; Farhat ,R.(2000) : Cytokine production by maternal lymphocytes during normal human pregnancy and in unexplained recurrent spontaneous abortion. Hum Reprod., 15: 713-
718.
10.Al-Zubadi, A.A.A. (2013). Interferon-gamma (IFN-g) gene Polymorphism as a Predisposing factor to Tuberculosis in
Babylon Province-Iraq. Ph.D. Thesis, college of Medicine. Babylon University.
11.Prigoshin, N; Tambutti, M; Larriba, J.; Gogorza, s and Testa, R. (2004). Cytokine Gene Polymorphisms in Recurrent
Pregnancy Loss of Unknown Cause. Am. J. Reprod. Immunol., 52 (1):36-4.
12.Kamali-Sarvestani, E.; Zolghadri, J.; Gharesi-Fard, B.; Sarvari, J. (2005). Cytokine gene polymorphisms and susceptibility to recurrent pregnancy loss in Iranian women. J Reprod Immunol., 65(2):171-8.
13.Pravica, V.; Perrey, C.; Stevens A.; Lee J.H. and Hutchinson I.V.(2000). A single nucleotide polymorphism in the first intron of the human IFN-D gene: absolute correlation with a polymorphic CA microsatellite marker of high IFN-y production. Hum. Immunol. 61(9):863-866.
14.Parveen, F.; Shukla, A. and Agarwal, S. (2013). Cytokine gene polymorphisms in northern Indian women with recurrent miscarriages. Fertil. and Steril. , 99(2): 433-440.
15.Daher, S.; Shulzhenko, N.; Morgun, A.; Mattar, S.; Rampim, G.F.; Cumano, L.; DeLima, M.G. (2003). Association between cytokine gene polymorphisms and recurrent pregnancy loss. J. Reprod. Immunol., 58: 69-77.
16.Babbage, S.J.; Arkwright, P.D.; Vince, G.S.; Perrey, C.; Pravica, V.; Quebery, S., Bates, M.; Hutchinson, I.V. (2001). Cytokine promoter gene polymorphisms and idiopathic recurrent pregnancy loss. J. Reprod. Immunol., 51: 21-27.
17.Bombell, S. and McGuire, W. (2008). Cytokine polymorphisms in women with recurrent pregnancy loss: meta-analysis. Aust. N Z J Obstet. Gynaecol., 48(2):147-54.
18.Choi, Y.K. and Kwak-Kim, J. (2008). Cytokine gene polymorphisms in recurrent spontaneous abortions: A comprehensive review. Am. J. Reprod. Immunol. 60(2):91-110.
19.Hassan, M.I.; Aschner, Y.; Manning, C.H. Xu, J. and Aschner, J.L. (2003). Racial differences in selected cytokine allelic and genotypic frequencies among healthy, pregnant women in North Carolina. Cytokine, 21(1):10-6.
20.Pravica, V.; Asderakis, A.; Perrey, C.; Hajeer, A.; Sinnott, P. J. and Hutchison, I.V. (1999). In vitro production of IFN- gamma correlates with CA repeat polymorphism in the human IFN-gamma gene. Europ. J. Immunogenet. 26: (1-3).
21.Abdullah, G.A. and Mahdi, N. K. (2013). The Role of Cytokine Among Women with Spontaneous Miscarriage. Med. J.of
Islamic World Aca. Sci., 21(3), 119-124.
22.Bakir, W.A.; Abdul-gany, Z.S. and faeeg, A. (2010). The Role of IL-6, IL-10 and IFN- γ mRNA in Women with Recurrent
Abortion. Iraqi Journal of Cancer and Medical Genetics, 3(1):46-53.
23.Bates, M.D., Quenby, S., Takakuwa, K., Johnson, P.M. and Vince, G.S. (2002). Aberrant cytokine production by peripheral blood mononuclear cells in recurrent pregnancy loss? Hum. Reprod., 17(9):2439-2444.
24. Darweesh, M.F. (2008). Immunological study for Listeria monocytogenes isolated locally. Ph.D. Thesis, college of science. Baghdad University.
25.Bradley, L.M.; Dalton, D.K. and Croft, M. A. (1996). direct role for IFN-γ in regualtion of Thl cell development. J. Immunol., 157: 1350-1358.
26.Abdul Mohymen , N. and Hussain, A. (2008). Detection of IL-10, IFN-γ and IL-8 in sera of patients with recurrent spontaneous abortion. IRAQI J MED. SCI., 6(2):58-65.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014
ISSN 2229-5518
1302
27.Ho, S., Winkler-Lowen, B., Morrish, B., Dakour, J., Li, H. and Guilbert, L.J. (1999) The role of Bcl-2 expression in EGF
inhibition of TNFD/IFNDD induced villous trophoblast apoptosis. Placenta, 20: 423-430.
28.Knofler, M., Mosl, B., Bauer, S., Griesinger, G. and Husslein, P. (2000). TNFa/TNFRI in primary and immortalised first trimester cytotrophoblasts. Placenta, 2:525-535.
29.Smith, S., Francis, R., Guilbert, L. and Baker, P.N. (2002) Growth factor rescue of cytokine mediated trophoblast apoptosis. Placenta, 23, 322-330.
30.Hussein, Y.M.; Ahmad, A.S.; Ibrahem, M.M. ; El Tarhouny, S.A.; Shalaby, S.M.; Elshal, A.S. El Said, M. (2009). Interferon
Gamma Gene Polymorphism as a Biochemical Marker in Egyptian Atopic Patients. J. Investig. Allergol. Clin. Immunol.,
19(4): 292-298.
31.Tso, H.W.; Chong, W.P.; Tam, C.M.; Chiang, A.K.S. and Lau, Y.L. (2005). Association of interferon gamma and interleukin 10 genes with tuberculosis in Hong Kong Chinese. Genes and Immunity, 6:358-363.
32.Baud'huin, M.; Lamoureux, F.; Duplomb, L.; Rédini, F.;& Heymann, D. (2007). "RANKL, RANK, osteoprotegerin: key partners of osteoimmunology and vascular diseases". Cell Mol. Life Sci., 64 (18):2334-2350.
33.Livolsi, A.; Busuttil, V.; Imbert, V.; Abraham, R.T. and Peyron, J.F. (2001). "Tyrosine phosphorylation-dependent activation of NF-κB. Requirement for p56 LCK and ZAP-70 protein tyrosine kinases". Eur. J. Biochem., 268 (5): 1508-15.
IJSER © 2014 http://www.ijser.org