Anode Reactions: 2H 2 => 4H+ + 4e-
Cathode Reactions: O 2 + 4H+ + 4e- => 2 H2O
Overall Cell Reactions:2H 2 + O2 => 2 H2O + Heat + Electricity
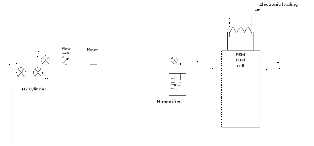
Experimental setup
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013
ISSN 2229-5518
1621
Sanjeev Doijode1 , B.P. Yadav2 , B Sudheer Premkumar3
JNTU research scholar, Hyderabad, Department of Mechanical Engineering ,REC Bhalki, 585328
Sandoij@yahoo.com
Principal, SPR College of Engineering and Technology , Ankushpur byadavp@yahoo.co.in
Prof, Department of Mechanical Engineering , JNTU, Hyderabad, 500085 bsudheerpk@yahoo.co.in
Proton exchange membrane fuel cells are getting great atten tion as energy source due to its high efficiency, low emission and high power density. The main objective of this paper is to study the performance o f PEM fuel cell with variation in temperature of fuel. Initially the PEM fuel cell was
tested with atmospheric condition & further it is
Reaction are as follows: -
Anode Reactions: 2H 2 => 4H+ + 4e-
Cathode Reactions: O 2 + 4H+ + 4e- => 2 H2O
Overall Cell Reactions:2H 2 + O2 => 2 H2O + Heat + Electricity
Experimental setup
tested with variation in fuel temp of 40 0 c & 600 etc. Accordingly the behavior of PEM fuel cell is studied and results are plotted on the graph.
The proton exchange membrane fuel cell is a device which converts chemical energy of fuel and oxidant into electrical energy and heat energy without classical combustion. The PEM fuel cell work with polymer el ectrolyte in the form of thin permeable sheet (Naffion membrane). This membrane is small and light and it works at room. Temperature. To speed the reaction a platinum catalyst is used on both sides of membrane. Next to catalyst anode and cathode (GDL-gas diffusion layer) of about 235-427 micrometer is provided on both side. The total assembly is known as MEA i.e.Membrane electrode assembly. The MEA is provided with flow field pattern on either side. The hydrogen is supplied from anode side and oxygen is supplied on cathode side. Hydrogen atoms are ionised at the anode and the positively charged proton diffuse through one side of the porous membrane and migrate towards the cathode. The electron pass from the anode to the cathode through an exterior circuit and provide electrical power along the way. At the cathode the electron, hydrogen proton and oxygen from the air combine to form water. The heat energy also produces during the process.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013
ISSN 2229-5518
1622
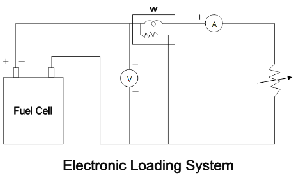
The experimental test rig consists of self breathing rig PEM fuel cell as shown in the figure. The hydrogen fuel will be supplied towards the anode side. A hydrogen cylinder with a pressure regulator (2 in no.) will govern the supply of hydrogen to the PEM fuel cell .For 12 w fuel cell the inlet pressure of hydrogen is maintained between 0.4 to 0. 5 bar. In the path of hydrogen flow a flow meter is installed to know the quantity of hydrogen being supplied to PEM fuel cell. The flow meter is of rotameter type having the graduation from 25ml to 275 ml /min in a step of 25 ml. Next to flow meter an electric heater is installed in a path and its temperature can be changed with the variable input.
The fuel cell will be tested in different ways. In this test the fuel cell is tested with change in fuel temperature i e ( hydrogen) .Initially it is constantly supplied to PEM fuel cell at room tmperature from anode side and later its temperature is changed to 40 c and 60 c..Since, the PEM fuel cell is self breathing hence it is not necessary to measure oxygen quantity. It takes as per its requirement .Due to this PEM fuel cell produces maximum power output.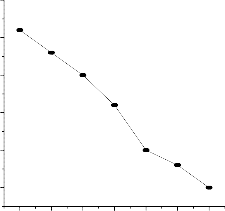
4.0
3.5
3.0
2.5
2.0
1.5
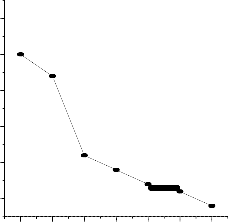
4.0
3.5
At 400 C Fuel Temp.
0.02 0.04 0.06 0.08 0.10 0.12 0.14
current (A)
At 600 C Fuel Temp.
IJSER
electronically with rheostat and ammeter by changing
the resistance from 10 ohm to 200 ohm, Accordingly the variation in the voltage and current output is recorded. It may be also tested with variation in fuel ie in a step of 25ml/min to 275 ml/min . From the observation table different graphs have been plotted which is shown below accordingly .
3.0
2.5
2.0
1.5
4.0
3.5
At Room Temp.
0.02 0.04 0.06 0.08 0.10 0.12 0.14
current (A)
3.0
2.5
2.0
1.5
0.02 0.04 0.06 0.08 0.10 0.12 0.14
current (A)
It is observed from the a bove experimentation and plots that the variation in input temperature of fuel has very little effect on the performance of fuel cell. At room temperature the performance shows positive response whereas with change in temperature of the fuel at 40 c and 60 c has poor performance.
1. Ru – jun yu and others, fabrication of support tubular proton exchange membrane for fuel cell, journal of fuel cell science and technology, vol 4, 2007 PP – 522-524
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013
ISSN 2229-5518
1623
2. Zetao xia and others, Development of cylindrical shape self breathing mini fuel cell stack, journal of fuel cell science and technology vol 5, 2008 PP - 0110121-1-7
3. T Henri ques and others, Increasing the efficiency of portable PEM fuel cell by altering the cathode channel geometry, Applied energy vol 87, 2010 PP-1400 to 1409
4. Yongping Hou and others, an experimental study on the dynamic process of PEM Fuel cell stack voltage, Renewable energy vol 36, 2011, PP-325 - 329
5. Comparative study of different fuel cell technologies. Renewable and sustainable energy review 16, 981 -989, 2012
6. Component failure analysis from stationary PEM fuel cell demonstration Journal of fuel science and technology (ASME) vol – 9
051007 2012
7. An empirical stationary fuel cell model using limited experimental data for identifi cation Journal of fuel science and technology (ASME) vol –9 061001 2012
8. Stack operation using composite membrane electrode assembly at 120 0C. Journal of fuel science and technology (ASME) vol – 9
031005 2012
9. A new complete design for air breathing PEM fuel cell aided by rapid prototyping. Journal of fuel science and technology (ASME) vol – 9
014502 2012
IJSER © 2013 http://www.ijser.org