The research paper published by IJSER journal is about Synthesis, polymeric and studied new derivative 2-aminobenzothiazole imide 1
ISSN 2229-5518
Synthesis, polymeric and studied new derivative
2-aminobenzothiazole imide
Mohmoud A-AL-Issa1, Entesar O.A-Temimi2 and Hind F-AL-Gburi1
1Department of Chemistry, College of Science for Woman, University of Baghdad, Iraq
2Department of Chemistry, College of Science, University of Baghdad, Iraq
Abstract— Prepared new substituted and unsubstituted poly imides compounds from using reaction of acrylol chloride with different amides (aliphatic, aromatic) in the presence of a suitable solvent and amount triethyl amine (Et3N) with heating. The Structure confirmation of all polymers were provide using FT-IR,1H-NMR,13C-NMR and UV spectroscopy. Thermal analysis (TG) for some polymers confirmed their thermal stabilities. Other physical properties including softening points, melting point and solubility of the polymers were also measured.
Index Terms— Synthesis, polymeric, aminobenzothiazole
—————————— ——————————
1 INTRODUCTION
enzothiazole is a privileged bicyclic ring system. Due to its potent and significant biological activities it has great pharmaceutical importance, hence, synthesis of this com- pound is of considerable interest[1]. Also it has been found that 2-substituted benzothiazole have good potential to exhibit anti-cancer activity[2,3,4]. Acryloyl chloride are polymerized easily to linear polymer if they are carefully protected from oxygen and water before and during polymerization of acry- loyl chloride is readily accomplished in bulk at room tempera- ture by exposure to ultra-violet light in quartz tubes[5]. Poly imides have become one of the most important and versatile classes of high performance polymer[6] due to excellent me- chanical and thermal properties. Aromatic heterocyclic imides of important in preparing of most commercial polyimides. In
2001[7] advanced research was published in the field of aero- space application. The research was carried by Connell. J. W and Watson KA. Through the preparation a space environ- mentally stable polyimides with a unique combination of properties including pale color in thin films, atomic oxygen resistance. UV- radiation resistance, solubility in organic sol- vents in the imide from high glass transition Tg temperature and high thermal stability by reacting novel aromatic diamine, bis(3-aminophenyl)-3,5-di(triflouromethyl) phenyl phosphine oxide with various aromatic di anhydrides in a polar aprotic solvent. Potentially use full in a variety of applications of space craft such as thin film membranes an antennae second surface mirrors, thermal, optical cotings and multi-layer ther- mal insulation materials blanket.
1.1 EXPERIMENTAL:
Melting points were recorded by using Gallen Kamp MFB-600 capillary melting point apparatus. FT-IR spectra were record- ed using solid KBr discs by testing Shimadzu FT-IR 8000 se- ries Fourier transform, infrared spectrophotometer. Thermal analyses were performed using thermal analysis system con- sisting from TG50 Shimadzu, Japan. Ibn Sina in Iraq. 1H-NMR and 13C-NMR spectra. Company Bruker, model ultra shield
300MHz, were made at the chemistry department, Al-Albyt university, Jordan.
A) Preparation of 2-aminobenzothiazole Derivatives[8,9] In a 250 ml round bottomed flask equipped with a magnetic bar stirrer and dropping funnel, a solution of bromine (1.2 ml) in glacial acetic acid (75 ml) was allowed to run through the dropping funnel drop wise during 30 min. To a mixture of Para substituted aromatic amine (0.03 mol) and ammonium thiocya- nate (0.1 mol) in 150 ml glacial acetic acid with stir- ring. The mixture was stirred for 1 hr., then diluted with water and neutralized with solid sodium hy- droxide. The precipitated substance was filtered, tri- turated and recrystallized from a suitable solvent to obtain 2-amino benzothiazole derivatives. The physi- cal properties and starting material of the synthesized compounds are given in Table (1).
B) General Procedure Preparation of 2-[N-(sub ben- zoyl)amidyl sub benzo thiazole]
In a round bottom flask equipped with a magnetic
bar stirrer and reflux condenser was placed a mixture
of sub-benzoyl chloride (0.06 mol) and (0.06 mol) 6-
sub-2-aminobenzothiazole with (3) drops of triethyl
amine (Et3N) in (25 ml) of suitable solvent (Benzene)
and refluxed (1-3) hrs. After the solvent was removed
and recrystallized from ethanol.
C) General Procedure Preparation of Poly 2-(N-acryl-N-sub benzoyl) imidyl Substituted Benzothiazole
In a round bottom flask equipped with a magnetic bar
stirrer was placed a mixture of poly (acryloyl
chloride) (0.06 mol) and (0.06 mol) of 2-N-sub amidyl-
sub benzothiazole with (1 ml) of triethyl amine (Et3N)
in (25 ml) of suitable solvent (THF or DMF) and ref-
luxed for (5-7) hrs. After cooling and removed the
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Synthesis, polymeric and studied new derivative 2-aminobenzothiazole imide 2
ISSN 2229-5518
solvent. The solid separated was filtered and purified by dissolving at DMF or DMSO and re precipitating from water or acetone. This procedure was applied on compounds [ ] as is shown in Table (1). All physical properties are listed in Table (1).
2 RESULTS AND DISCUSSION
2.1 PREPARATION OF [2-(N-BENZOYL) AMIDYL SUB BEN- ZOTHIAZOLE]
All compounds characteristics (m.p, FT-IR, UV and C.H.N). Preparation of poly [2-(N-acryl-N-benzoyl) imidyl substituted benzothiazole. Table (1) polymer was prepared by the reaction of poly (acryloyl chloride) with different amides [A] in pres- ence of triethyl amine (Et3N) as a catalyst [10].
The mechanism of reaction involves a nucleophilic attack on the carbonyl as in shown below [11].
Structures confirmation of all prepared polymer proved using







FT-IR, UV, 1H-NMR, 13C-NMR, TG and physical properties including softening point, solubility, Tg and percent conver- sion of the polymers. FT-IR spectrum of compounds showed the same bands appearance. Stretching band at 1670cm-1 (C=O), (2800-2920)cm-1 (C-H) aliphatic, (1590-1630)cm-1 (C ----C) and (3010-3100)cm-1 (C-H) aromatic as shown Ta- ble (2).1H-NMR spectrum of polymers [7,9,11] showed the signals at (1.021) (t, 2H), (3-3.9) (m, 1H,) while a signal at (7-8) (d or m, 1H, Ph-H) for compounds[4,6,8] and at 1.02 (s,



3H, Ph-CH3), as show listed in Table (3). The 13C-NMR spec- trum of compound showed the signal at (170-180) ppm for carbonyl group (C=O) and appeared at (120-140) ppm and appeared at (20-50.48) ppm. UV spectrum of compounds ab- sorption max at 300nm and 380nm which attributed to ( *) and (n *) transitions. See Table (4). TG analysis pro- vides a change in the mass of the polymer during heating. The thermal analysis was carried out at temperatures (20-40)ºC with heating rate 20.0 ºC/min. in N2 atmosphere. Thermal stability of the product was estimated from TG and DTG thermo grams. See Table (5), it was found that the prepared polymers high stability, resists to isomerization by heat, light or using acidic or basic solution[12,13].
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Synthesis, polymeric and studied new derivative 2-aminobenzothiazole imide 3
ISSN 2229-5518

Table (1): Physical properties of the poly imide
Com p. No. | Compound structure | Conver- sion % | Soft- ing point C | Colour | Solvent used in reac- tion |
1 | 
| 85.7 | 182- 192 | Brow- nish yellow | THF or DMF |
2 | 
| 84.9 | 170- 185 | Brow- nish yellow | THF or DMF |
3 | 
| 87.3 | 140- 165 | Light yellow | THF or DMF |
4 | 
| 80 | 135- 148 | Light Brown | THF or DMF |
5 | 
| 79.4 | 150- 163 | Light Brown | THF or DMF |
6 | 
| 84 | 190- 202 | Light yellow | THF or DMF |
7 | 
| 82 | 130- 142 | Light yellow | THF or DMF |
8 | 
| 84 | 210- 220 | Brown | THF or DMF |
9 | 
| 80 | 140- 153 | Brown | THF or DMF |
10 | 
| 81 | | Light yellow | THF or DMF |
11 | 
| 80 | 190- 200 | Light green | THF or DMF |
12 | IJS http:// | 85 ER © 2012 www.ijser.org | 130- 140 | Yellow | THF or DMF |
The research paper published by IJSER journal is about Synthesis, polymeric and studied new derivative 2-aminobenzothiazole imide 4
ISSN 2229-5518
Table (2): FT-IR spectra of the prepared heterocyclic polyimide
Comp.
FTIR spectral data cm-1
No. Compound structure
(C=
O)
imide
(C-
N)
imide
(C=C)
aromatic
(C-H)
aliphatic
(C-H)
aromatic
(C=N)
imine
(C- Others
S)
1 1697 1442 1600 2947
2978
3055 1546 640 -
2 1678 1442 1600
2947
2978 3035 1519 613
C-NO2
1346
3 1693 1400 1600 2943
2978
3132 - - -
4 1678 1441 3174
1604
2943
2978
3174 - 613 -
5 1678 1446 1604 2943
2978
3186 - 609 C-NO2
1346
6 1698 1400 1589 2920
2940
3070 - 610 C-NO2
1350
7 1680 1400 1600 1920 3005 - 610 C-NO2
1360
8 1690 1410 1602 2990 3030 - 620
C-NO2
1340
9 1691 1392
1585
1600
2837
2900 3097 1591 584
C-Cl, C-NO2
1037, 1444
10 1701 1300
1554
1581
2897
2823 3070 1554 651
C-Cl
1072
11 1681 1442 1585 2900
2950
3100 1539 610 C-Cl
1034
IJSER © 2012
http://www.ijser.org
2990
C-Cl
1010

12 1690
1390
1600
2980
3010 1530 610
C-NO2
1360
The research paper published by IJSER journal is about Synthesis, polymeric and studied new derivative 2-aminobenzothiazole imide 5
ISSN 2229-5518











Table (3): The 1H-NMR chemical shifts of some of the prepared poly heterocyclic imides
Comp . No. | Compound structure | Chemical shifts |
4 | | 1.03(s,3H4); 3-3.5 (t, 2H2, H2C CH n ; 4.2(m, 1H1), ; 7-8 (m, HAr) H2C CH n |
6 | | 1.08(t, 2H6,  H2C CH ); 2.6(3H1); H2C CH ); 2.6(3H1); n 3.4(m, 1H5, ); 7.4(d, 1H2, HAr); 7.9(d, 1H3, HA Hr2)C;  C7H.9(sn, 1H4, HAr) C7H.9(sn, 1H4, HAr) |
7 | | 1.18(t, 2H2,  H2C CH );n H2C CH );n 2.7(m,1H1, ); 7.5(m,1H8, H2C ; CH nd, 1H7, 1H6, HAr); HAr) 7.8( 8(d, 1H10, 1H9, HAr); 8.1(d,,1H5,HAr); 8.26(d, 1H4, HAr); 8.5(s, 1H3, HAr) |
8 | | 1.03(t, 2H6, H2C CH n ); 3.0(s,2H1) 3.5(m,1H5, H2C CH n ); 7.4(d, 1H3,HAr) 8.1(d,H2, HAr); 8.3(s,1H4, HAr) |
9 | | 1.2(t,2H2, H2C CH n ); H2C CH n ); 3.3(m,1H1, H C CH ); H C CH ); (7.3-7.8)(d, H2 H , n r); 7.8 (t, 1H7, 1H8, HAr); 9, 6 HA 8.2(d, H5, HAr); 8.3(d, H4, HAr); 8.5(s, H3, HAr) |










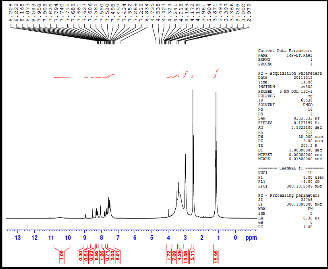
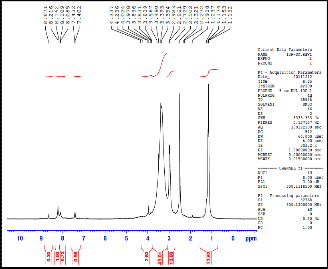
Fig.(1): 1H-NMR for compound [8] Fig.(2): 1H-NMR for compound [9]
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Synthesis, polymeric and studied new derivative 2-aminobenzothiazole imide 6
ISSN 2229-5518
Table (4): UV spectra of the prepared heterocyclic polyimide

http://www.ijser.org
The research paper published by IJSER journal is about Synthesis, polymeric and studied new derivative 2-aminobenzothiazole imide 7
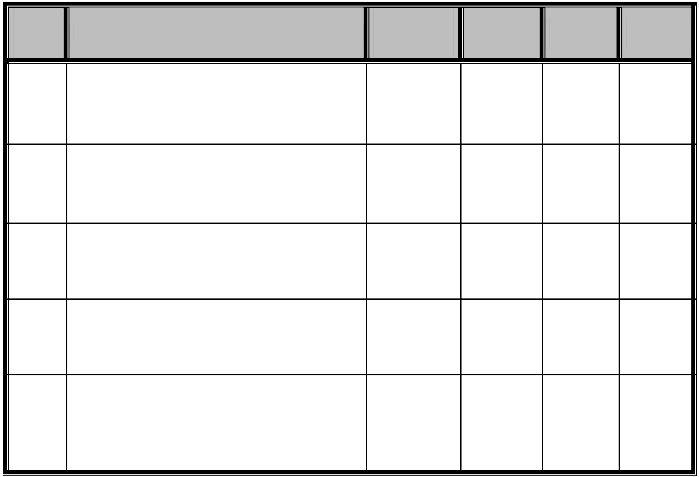
ISSN 2229-5518
Comp
.
No.
Compound structure
10% wt
Loss temp
ºC
50% wt
Loss temp
ºC
Peak 1
Temp ºC
Peak 2
Temp ºC
1 217.2 341.75 250 389
2 207.77 298.66 245.2 393.15
3 178 260 256.9 287.2
11 206.77 224.3 235 314.6
12 212.67 377.1 271.7 237.15
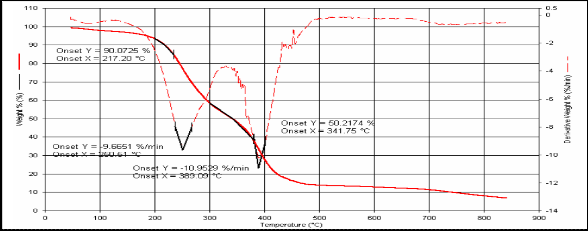
Fig.(3): TG for compound [1]
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Synthesis, polymeric and studied new derivative 2-aminobenzothiazole imide 8
ISSN 2229-5518
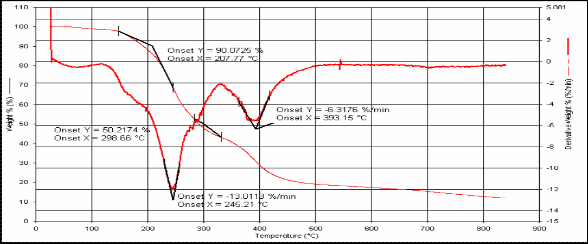
Fig.(4): TG for compound [2]
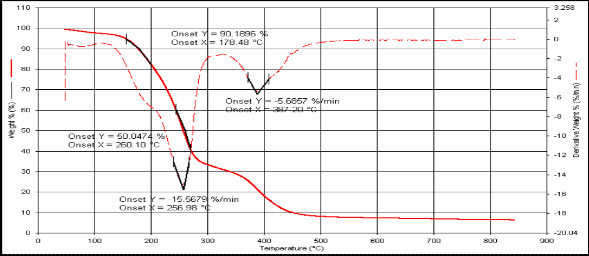
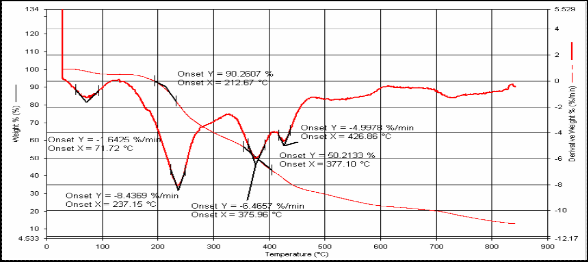
Fig.(5): TG for compound [3]
Fig.(6): TG for compound [11]
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Synthesis, polymeric and studied new derivative 2-aminobenzothiazole imide 9
ISSN 2229-5518
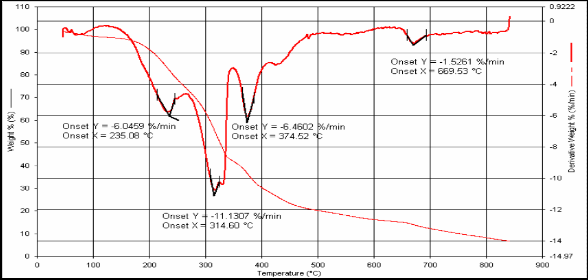
Fig.(7): TG for compound [12]
REFERENCES
1. A. Gupta and S. Rawat."Synthesis and cyclization of Bezo-
thiazole". J. of Current Pharmaceutical Research., 3(1), 13-
25 (2010).
2. S. R. Pattan and S. N. Narendra babu. "Synthesis and anti- fungal activity of 2-amino[5'-(4-sulphonyl benzylidene)- 2,4
-thiazolidene dione]-7-(sub)-6-fluro benzo thiazoles". Ind. J.
of Het. Chem., 11,333-334 (2006).
3. S. T. Huang and H. Igen. "Synthesis and anti-cancer evalu- tion of bis(benzimidazoles), bis (benzox- azoles)and benzothiazoles". Bio and Med. Chem., 146, 106-
109 (2006).
4. J. A. Khanam and P. Bags. "Anti-neoplastic activity of cop- per benzohydroxamic and complex against Ehrlich Ascites Carcinoma (EAC) in mice". Int. J. Pharm., 29(3), 157-160 (1997).
5. S. J. AL-Bayti. Ph.D. Thesis, College of Science for Women,
University of Baghdad (2009).
6. A. D. Bowens. Ph.D. Thesis, Faulty of the Virginia Institute
and State University (1999).
7. S. M. Hussain. Ph.D. Thesis, College of Science, University of Baghdad (2003).
8. C. G. Stuckwisch. J. Am. Chem. Soc., 71, 3417 (1949).
9. S. A. R. Mahdi. M.Sc. Thesis, College of Science, University of Baghdad (2008).
10. F. A. Carey and R.J. Sundber. “Advanced Organic Chemi-
stry Part A: Structure and Mechanisms”, 5th Ed., New York
(2007).
11. P. Crews, J. Rodriguez and M. Jaspars. “Organic structure analysis”, Oxford University Press, New York (1998).
12. U. V. Korshak. “The Chemical structure and thermal cha-
racteristics of polymer” (1969).
13. G. H. A. Al-Farajy, M.Sc. Thesis, College of Science, Univer- sity of Baghdad (2003).
IJSER © 2012
http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 3, Issue 8, August-2012 10
ISSN 2229-5518
IJSER lb) 2012
htt p://www .'lser. ora








