International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2949
ISSN 2229-5518
Synthesis of Pyridine and Derive Complexes Using Transition
Metals Complex Including Pd
Sachin Kumar1, Pushpendra Singh2, Kapil Sirohi3,Nempal Singh4
1. Dept. of Applied Sciences, Indraprastha Institute of Technology, Gajraula, U.P.
2. Dept. of Applied Sciences, Teerthanker Mahaveer University, Moradabad, U.P.
3. Dept. of Basic Education, Moradabad, U.P.
4. Dept. of Applied Sciences, Indraprastha Institute of Technology, Gajraula, U.P. Email: drsachingill@gmail.com, psalunaphys@gmail.com
Abstract
A development of prospective synthetic methods to produce Pyridine including those of complex structure will allow to makes the latter more available and hence Pyridine bases will be widely used in industry. In this paper the Hetrocyclization of Acetylenes with nit riles catalyzed by palladinium complex has been carried out. We have limited our aim to study the palladinium catalyzed activation of bifunctional vinyl halide a-Bromoacrylic amides with 1, 3 dienes and alkynes to form an unsaturated butyrolactums. The compounds so obtained is 1.1-(4- Methoxyphenyl)-3(1 phenyl-(E)- methylidene)-
2,3,3a,4,5,7a- hexahydro- 1H-2-indolone and 1N-(4-Methoxyphenyl)-2-bromo-3-phenyl-(Z)-2-
propenamide.
Keywords: palladinium complex, 1.1-(4- Methoxyphenyl)-3(1 phenyl-(E) - methylidene) - 2, 3, 3a, 4,
5,7a- hexahydro- 1H-2-indolone
1. Introduction
A Development of Prospective Synthetic and Methods to Produce Pyridine Including those of Complex Structure. In Synthesis Of Substituted Pyridine By Acetylene Hetrocyclization with Nit riles (RC=NCR=CH3, Ph, Ph, Ch2) In the presence of Co-Containing Complex Catalysts was published by Japanese Researches and the Carbon Hetro Bond Formation Reaction Catalyzed by the Transmission Metal Complex has been very attractive formation of Idols Aziridines and other Heterocyclic which one part of Biologically Interesting Products and the Synthesis of Pyridine Basis Including. Those of Natural Structure with the use of Metal Complex Catalyst .The Transition Metal (Co ,Ni,Cr ,Pd ,Zr ) and Rare Earth Elements Reactions of Hetrocyclazation of Acetylene, Liquids Phase Condensation of Aldehyde S with Amines, Linear and Cyclic Oligomerization of Vinyl Pyridines with Olefins Acetylenes Tertiary Alcohols to give Substituted Pyridines Quoins and Phenanthrolines of a structure. .
2. Experimental Details
Our aim is to study the Palladinium-Catalyzed Activation of Bifunctional Vinyl Halide. A Bromoacrylic Amide with 1, 3 Dina and Alkynes To Form An Unsaturated Butyrolactums Reaction Involved Formation of Oxidative Addition Complex with Vinyl Halide and then the in Sertion of 1, 3
Dines B/W The Carbon palladinium In Bonds Leading To P Allyl palladinium Bond Leading Complex
And The heteroatom nucleophile attack on the p-ally palladinium complex, which leads to expected lactums.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2950
ISSN 2229-5518
The synthesis of various Bromoacrylic amides and palladium catalyzed reaction of bromo acrylic amides were synthesized from the corresponding a- substituted anilines in the presence of triethylamine at room temperature.
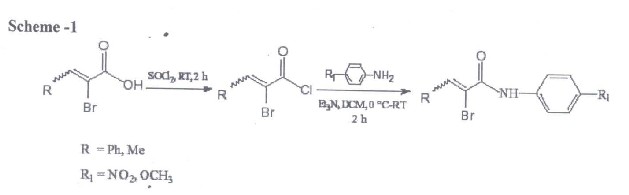
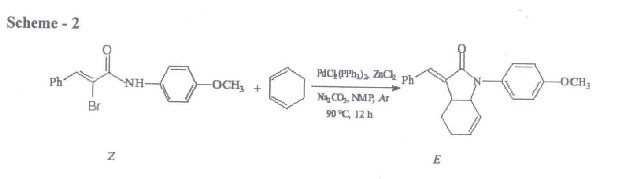
Initial study was on the reaction of 1N- (4 Methoxyphenyl)-2- bromo-3-phenyl-(E)-2-propenamyde with1,3 cyclohexadiene catalyzed by PdCl2 (PPh3)2 and co- catalyst, zinc chloride to yield the expected butyrolactums , 1 –(4-methoxyphenyl)-3-(1- phenyl-(E)-methylidene)-2,3,3a,4,5,7a-hexa-hydro1 H-2- indolone as shown scheme below;
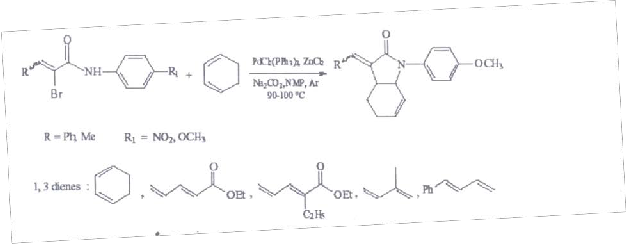
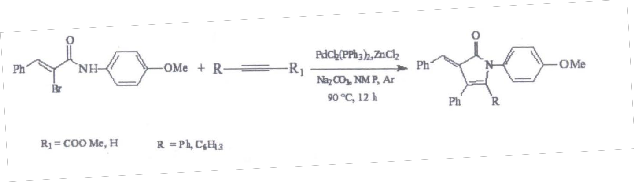
Similar reaction of various a-Bromoacrylic amides with different 1,3-dienes and alkynes were carried out in the presence of CrCl2(PPh3) and zinc chloride at 90-100 oC under argon atmosphere to yield the corresponding substituted butyrolactums in good yield( scheme-3 & 4). The result of reactions of a- Bromoacrylic amides with 1, 3 dienes are tabulated in third chapter.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2951
ISSN 2229-5518
Scheme-3
Scheme-4
General procedure for the palladinium- catalyzed reaction of a- Bromoacrylic amides with 1, 3-dienes.
A 25 mL RB flask equipped with a magnetic stirring bar, reflux condenser and argon balloon was
charged with a- Bromoacrylic amide (1 mmol), 1, 3- dienes (2 mmol), PdCl2(PPh3)2 (0.07 g, 0.1 mmol), sodium carbonate (0.275 g, 2 mmol), zinc chloride (0.067g, 0.5 mmol) and degassed N-
,methylpyrrelidone (4 mL). the reaction mixture was flushed with argon thrice an d allowed to stir at 90 oC for 2-48h. The reaction mixture was neutralized with dil. HCl and the product was extracted with ethyl acetate (3 – 5 mL). The combined organic layer was dried over anhydrous sodium sulphate and concentrated under reduced pressure to give crude product. The crude product on silica gel column chromatographic purification using a mixture of petroleum ether and ethyl acetate gave the corresponding butyrolactums in moderate to good yield. The compound obtained is 1.1-(4-methoxyphenyl)-3-(1-
phenyl-(E)-methylidene)-2, 3, 3a, 4, 5, 7a-hexahydro-1H-2-indoone.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2952
ISSN 2229-5518
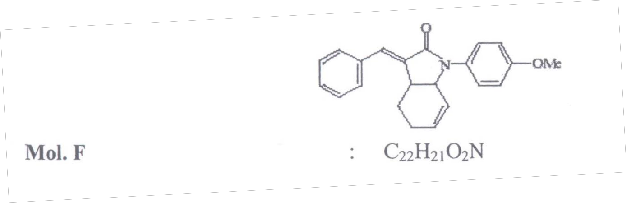
M.P : 135 - 1370C
IR (Nujol) : 3018, 2935, 1681, 1645, 1608, 1290, 1247, 1033, 692,cm-1
1 H NMR (200 MHz, CDCL3) : δ 7.6 (d,J = 4.0 Hz, 1H), 7.55 - 7.4 (m, 5H), 7.3 (δ, J = 8.0 Hz,2H)
6.95 (d, J= 8.0 Hz, 2H), 6.15 – 6.05 (m, 1H) 3.8 (s,3H),
3.65-3.50 (m,1H), 2.25 – 2.0 (m, 3H), 1.6 1.45 (m, 1H)
13 C MNR (50.32 MHz, CDCl3) : δ 168.65, 158.19, 136.52, 135.60, 133.25,
130.00, 129.77, 129.13, 126.63, 123.27, 114.64, 55.76, 54.77,
36.51, 24.45, 23.93.
Mass (m/z) : 331 (M+, 100), 302 (15), 212(18), 179 (12), 165 (22),
134 9), 115 (37) , 91 (24),
3. Conclusions
A new and novel methodology was developed towards the synthesis of a, b-unsaturated butyrolactums by the palladinium catalyzed reaction of a-bromo acrylic amides with 1, 3 dienes and
alkynes.
References-
1. Shrauzer, G.N. Chem.Ber.1961, 94, 1403.
2. Funk, R.L., Vollhardat, K.P.J.C.J.Am.Chem.Soc.1977, 99, 5483.
3. Iwashita, Y.,Tamura, F.Bull.Chem.Soc.Jpn.1970,43,1517.
4. Wilke G.Pure Appl.Chem.1978, 50,677.
5. Volhardat, K.P.C, Bergman.R.G.J.Am Chem.Soc.1974, 96, 4996.
6. Hillard, R.L., Vollhardat K.P.J. Am.Chem.Soc.1977, 99, 4058.
7. Wakatsuki, Y, Yamazki, H.Tetrahedron Let.1973, 3383.
8. Bonnemann, H.Angew, Chem1985, 97,264.
9. Bonnemann, H.Angew, Chem.1978, 90517.
10. Volhardat, k.p.c.angew.chem.1984, 96,525.
IJSER © 2013 http://www.ijser.org




