International Journal Of Scientific & Engineering Research, Volume 5, Issue 3, March-2014
ISSN 2229-5518
885
Synthesis of Hydrous Aluminum (III) -Iron (III)- Manganese (IV) Ternary Mixed Oxide for Fluoride Removal
Girma W. Woyessa, B.B.L.Srivastava, Ephrem G. Demissie
A b s t r a c t — Hydrous Aluminum (III)-Iron (III)-Manganese (IV) ternary mixed oxide was synthesized by Co-precipitation method. It was found that the Al: Fe: Mn sample with 75:15:10 w/v ratio exhibited maximum adsorption capacity for fluoride. The use of mixed oxide for investigating fluoride removal efficiency at varied condition showed that the reaction was pH sensitive and the optimum pH was between 6.00 and 7.00. The characterization studies on the adsorbent by XRD, SEM-EDX and FT-IR analysis for fluoride adsorption were carried out to understand the adsorption mechanism. The particles were irregular in shape, highly porous. FT-IR studies revealed a little change after fluoride adsorption and showed formation of new fluoride– oxygen bond on the adsorbent surface. Applicability of different sorption kinetics model; was studied. The pseudo-second order equation described all kinetic data very well. The Langmuir isotherm equation described the equilibrium very well. The thermodynamic analysis of the equilibrium indicated that the adsorption reaction of fluoride with hydrous Al-Fe-Mn mixed oxide system for water was endothermic and spontaneous in nature. The presence of
-3 showed significant effect on removal efficiency of Al-Fe-Mn oxide adsorbent.
PO4
Index Terms— Defluoridation, Batch adsorption, Hydrous Al-Fe-Mn mixed oxide
—————————— ——————————
.
1. INTRODUCTION
Ground water is usually the main source of fluoride intake. It was estimated that globally more than 70 million people are suffering from fluorosis [1].The high level of fluoride ion in drinking water is due to some geochemical reactions in sub-surface depending on the geological formation; fluorite and fluoroapatite minerals are hosted in the vein of almost all rocks in the subsurface [2]. The bicarbonate rich water from the topsoil when percolates through the mineral bed undergoes water-mineral interaction and releases fluoride due to some chemical reactions under geophysical conditions [3]. Fluoride contamination in ground water is a worldwide problem and its concentration in drinking water of many places exceeds the permissible values [4]. The maximum fluoride concentration of 1.5 mg/L has been recommended by world health organization as a standard internationally [5]. Fluoride is an essential mineral that in permissible guideline level is beneficial to mankind in dental protection and excessive intake leads to various disorder and diseases such as skeletal fluorosis[6] , brittle bones[7] and cancer (lung and bladder)[8].
————————————————
• Girma W. Woyessa, Haramaya University, Department of Chemistry, p.o.box 74, Dire Dawa, Ethiopia E-
mail: abelgirma827@gmail.com
• B.B.L.Srivastava, E-Mail: bbl_mgpg@yahoo.in
• Ephrem G. Demissie Haramaya University, Department of Chemistry, p.o.box 74, Dire Dawa, Ethiopia, E-
mail :ephrem1977@gmail.com
Currently several methods have been employed to remove excess fluoride from aqueous solution, and these can be broadly divided in to three categories based on mechanism of fluoride removal such as adsorption [9] precipitation method [10] and membrane separation [11]. Cost, rejection and raw water characteristics are among factors influencing in selection membranes [11]. Removal of excess fluoride from water using precipitation method is mainly governed by solubility of the salt. The most common method is the precipitation of calcium fluoride where, lime is used to precipitate fluoride as calcium fluoride (CaF2 ).
The fundamental problem that exists using lime arises from the low solubility of calcium hydroxide which requires excess of the reagent for the removal of fluoride [10]. Defluoridation of aqueous solutions via adsorption has attracted much attention in recent times due to their chemically stability over a wide pH range, environmentally friendly, effective treatment in dilute solutions, effective for fluoride removal, high uptake capacity and economical alternative for removing trace metals [9]. Many adsorbents such as activated alumina [12], granular ferric hydroxides [13], aluminum hydroxide [14], clay [15] and fly ash [16] have been used for fluoride removal. Among them, activated alumina is the most commonly used adsorbent for water defluoridation but it’s disadvantage including aluminum dissolution and relative sorption capacity prevent it from wide applicability[13].
Studies were reported on iron containing different mixed
metal oxide for their adsorption capacity for fluoride pollutants from water such as, Mn-Ce-oxide [17], hydrous iron (III)-Chromium (III) mixed oxide [18], hydrous
IJSER © 2014
http://www.ijser.org
International Journal Of Scientific & Engineering Research, Volume 5, Issue 3, March-2014
ISSN 2229-5518
886
aluminum oxides and alumina [23] and Fe-Al-Ce trimetal oxide [25] were synthesized and used for investigating the fluoride removal efficiency. Mixed metal oxide treatment systems have a potential advantage in terms of adsorption capacity and stabilization of the sorbent–fluoride product[7]. Therefore, in this study, the sorption feasibility of mixed ternary Al-Fe-Mn mixed oxide has been assessed for fluoride removal from aqueous solution.
2. MATERIALS AND METHODS
2.1Chemicals
1.6 g adsorbent, and the flasks were shaken at 150 rpm in a rotary shaker at 25oC for 3 h. After the adsorption experiments, the adsorbent was separated from the solution by a filter paper with a 0.20µm pore size and the residual fluoride concentration in solution was measured by ion meter equipped with fluoride ion selective electrode (Jenway,UK). All the experiments were performed in triplicate and the mean values were used and their standard deviations were calculated.
The adsorbed fluoride was computed as follows:
Aluminum nitrate dodeca hydrated,(Al(NO3 )3 .12H2 O,96%)
, Iron (II) nitrate hepta hydrated (Fe(NO3 )2 .7H2 O, 99%), manganese (II) nitrate hepta hydrated (Mn(NO3 )2 .7H2 O,
98%,) sodium fluoride (NaF,99%) were purchased from

% Adsorption = CO − Ce × 100%
CO
(C − C )

= o e ×
(1)
(2)
BDH chemical company, England and all were of analytical
grade.
2.2 Synthesis of hydrous Al-Fe-Mn mixed oxide
To synthesize nano sized Fe-Al-Mn ternary mixed oxides, co precipitation method was used. (Al(NO3 )3 .12H2 O, (Fe(NO3 )2 .7H2 O, (Mn(NO3 )2 .7H2 O, were dissolved in de- ionized water to form a mixed solution at a predetermined percentage of 75%, 15%, and 10% w/v respectively. NaOH(6M) was added slowly with mechanical agitation(250rpm) until the pH of the solution including the gel type precipitate reached 6.0-7.0. The precipitate was aged with the supernatant liquid for 24 h. The filtered precipitate was dried at 60-70oC for 12 h in air oven. The dried product was heat treated at 300oC for 3 h in to a muffle furnace and grounded with an agate mortar, sieved for fraction with 250µm molecular sieve. The residual precipitates after treating at the temperature were labeled and used for further experiments. Energy dispersive electron microscopy (EDX) was conducted for elemental analysis of the oxides in the mixture.
2.3 Characterization of the sorbent
The physical and chemical characterization of mixed ternary Al-Fe-Mn oxide was carried out by scanning electron microscope (SEM), X-ray diffraction (XRD) and Fourier transforms infra-red spectroscopy (FT-IR). Energy dispersive X-ray analysis (EDX) was conducted for elemental analysis of the mixed Al-Fe-Mn oxide.
2.4 Batch adsorption studies
The effect of different parameters such as adsorbate concentration, dose of adsorbent, agitation speed, pH and presence of foreign ions were studied by varying any one of the parameters and keeping the other parameters constant. The sorption experiments were carried out in 500 mL conical flasks containing 250 mL of fluoride solution and
Defluoridation capacity V
m
Where Co and Ce are the concentrations of F- initially and at an equilibrium time t in mg/L, respectively and m is the dose of the adsorbent in g/L and V is the volume of the reaction mixture in (L).
3. RESULTS AND DISCUSSION
3.1 Elemental Analysis and characterization
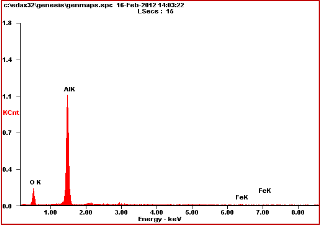
EDX analysis was performed to determine the elemental constituent of mixed Al-Fe-Mn oxide. The data shown in Fig.1 reveals that the wt % of Al: Fe: Mn oxide was found to be 71%, 12% and 10.30% respectively but the expected value is 75% for aluminum oxide,15% for hematite and 10% for manganese(IV) oxide. Thus, the EDX results suggest that the values obtained are in good agreement with the concentration at the synthesis procedure.
Fig.1.EDX spectra of Al-Fe-Mn mixed oxide (K: the K
shell,a:alpha X-ray line).
IJSER © 2014
http://www.ijser.org
International Journal Of Scientific & Engineering Research, Volume 5, Issue 3, March-2014
ISSN 2229-5518

3.2 XRD analysis
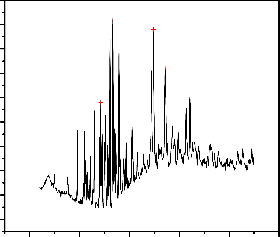
The diffraction peaks found at 32.18143o were the characterstic peaks of Fe2 O3 (Fig.2). The alumnium oxide exhibited the major peaks at 33.33964o indicating that Al2 O3 was the dominant species. Manganese oxide on the other hand was hardly detected in the XRD pattern and this may occur due to the presence of manganese oxide in the adsorbent might be insignificant or the formation Al-Fe-Mn solid solution might have forced the Mn species to occupy interstial holes created by the crystal lattices of Al2 O3 /Fe2 O [34].
887
1600
1500
33.33964
32.18143
49.6504
1300
1200
1100
1000
900
800
700
28.51675
54.30583
Fig. 3. SEM images for hydrous Al-Fe-Mn mixed metal oxide particles
3.4 FT-IR analysis
Results of the FT-IR study of Al-Fe-Mn mixed oxide showed sharp absorption peaks located 3,448 1,641, 1,165,
707 and 622 cm−1(Fig. 4). The absorption bands at 3448 and
1641 cm-1 are due to symmetrical stretching and bending vibration of the O-H group, respectively [23].
0 20 40 60 80 100
2θ(ο)
Fig.2.XRD patterns of Al-Fe-Mn mixed oxides at a temperature of 300oC
1641
After
SEM analysis shows irregularly distributed Al-Fe-Mn mixed metal oxide particles on the surfaces of the cells (Fig.3). The Al-Fe-Mn metal oxide particles were highly porous in shape, the larger Al-Fe-Mn oxide particles may be due to the aggregation of the smaller ones, as the result of SEM measurements.
374
622
707
1165
1250
1726
3448
3533
Before
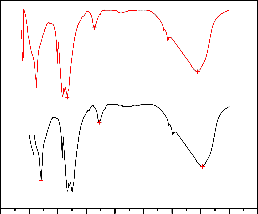
0 500 1000 1500 2000 2500 3000 3500 4000 4500
wavenumber (cm-1)
Fig.4. FT-IR spectra of the Al-Fe-Mn mixed oxide before and after adsorption
IJSER © 2014
http://www.ijser.org
International Journal Of Scientific & Engineering Research, Volume 5, Issue 3, March-2014
ISSN 2229-5518
888
The absorption peak at 622 cm-1 may be assigned to the symmetrical stretching M-O vibrations of the mixed metal oxides [25]. The appearance of a sharp peak at 374cm-1 corresponds to metal fluoride vibrations. The FT-IR spectroscopic study after sorption confirmed that the fluoride ion has a strong binding ability with the surface of Al-Fe-Mn mixed oxide particles, suggesting the formation of a layer covering the oxide particles and prevent
3.6. Sorption kinetics
The sorption rate constants of fluoride on nano-sized Al-Fe- Mn mixed ternary oxide were determined to understand the adsorption phenomena [32]. The sorption rate constants for fluoride were calculated by using the following Lagergren equation [33]:
k1
agglomeration.
log(qe − qt ) = log qe −

t
2.303
(3)
3.5 Effect of pH
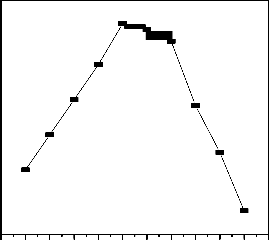
95


t = 1
qt k 2 qe

+ 1 t qe
(4)
90
85
80
75
70
65
60
55
1 2 3 4 5 6 7 8 9 10 11 12
pH of the solution
Where q t and q e are amount of fluoride adsorbed at a time t
and equilibrium (mg/g) respectively, k2 is the rate constant(g mg-1 min-1), t is the stirring time (min), k2 which is (slope2/intercept) can be determined from plotting t/qt against t based on above equation and the value of q e is
1/slope. The Coefficient of correlation for the first-order-
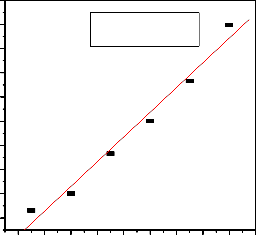
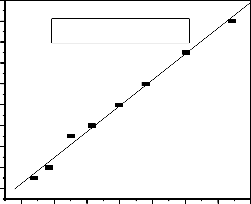
kinetic model and pseudo second-order reaction rate model was 0.9714 and 0.9892 respectively (Fig.6a and b). The theoretical equilibrium capacities q ecal (10.01 mg/g) fit well with the experimental data with q e (9.78 mg/g) as shown in table 2.Thus, the study indicated that the pseudo-second- order model better represents the fluoride adsorption kinetics (R2=0.9892), suggesting that more of the adsorption process might follows second order chemisorptions process.
0.2
mixed oxide at 25 ± 2 0C
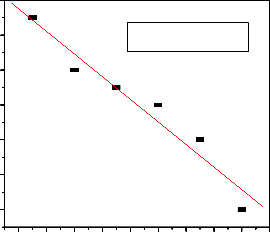
Hydrogen ion concentration in the adsorption is considered as one of the most important parameters that influence the adsorption behavior of metal ions in aqueous solution. The fluoride uptake capacity of Al-Fe-Mn mixed oxide is more favored in the pH range of 6 to 8 (Fig. 5). The sorption of fluoride increases with increased pH, reaching maximum at
6.02, and then decreases with further increase in pH.
Initially, as the pH increased from 2 to 7, the fluoride sorption increases due to the fact that for pH less than 7, the surface of Al-Fe-Mn mixed oxides becomes positively charged and attracts negative fluoride anions and becomes maximum at pH 6.02. On the other hand, the decrease in the sorption capacity at pH greater than 6.02 can be
0.0
-0.2
-0.4
-0.6
-0.8
-1.0
log(qe- qt)=0.28-.0064(t) R2=0.9714
20 40 60 80 100 120 140 160 180 200
t(min)
attributed to the competition for the active site by OH- ions
and or the development of negative charge on the adsorbent surface that makes repulsion between the adsorbate and the OH- ions [17].
Fig.6. (a) Pseudo first order plot.
IJSER © 2014
http://www.ijser.org
International Journal Of Scientific & Engineering Research, Volume 5, Issue 3, March-2014
ISSN 2229-5518
889
18
16 t/q =-3.5233 +0.1022(t) R2 = 0.9892
14
12
10
8
sorption capacity of sorbent, Ce is the equilibrium concentration of fluoride (mg/L) and b (L/mg) is Langmuir isotherm constant (mg/L), Kf is a measure of adsorption capacity for Freundlich model and 1/n is the adsorption intensity. The Freundlich isotherm constants 1/n and Kf can be calculated from the slope and intercept of the plot log q e vs log Ce . The values of 1/n (0.735) lying between 0 and 1 and Kf (6.605) between 1 and 10 indicated that the conditions were favorable for adsorption [35].
6 0.30
4 0.28
2 0.26
Ce/qe=0.24921+0.02307Ce
R2 = 0.9963
0
20 40 60 80 100 120 140 160 180 200
t(min)
0.24
0.22
0.20
0.18
Fig.6. (b) Pseudo second order plot for fluoride sorption
0.16

Table 1: Summary of the Pseudo first order rate constants and correlation coefficients
0.14
0.12
0 2 4 6 8 10 12 14
Adsorbate
(mg/L)

20 mg/0.8 g
k1 (min-1)
0.0064
Rate equation
log (q e -q t ) =
0.28-0.0064t
q e (mg/g)
3.57
R2
0.9714
Ce
Fig.7. (a) Adsorption Langmuir isotherm plot

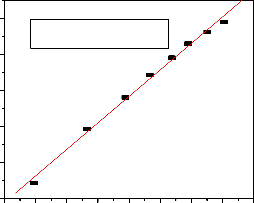
Table 2: Summary of the Pseudo second order rate constants and correlation coefficients
Adsorbate
k2 ( g min-1 mg-1)
Rate
q e R2
1.4
logq = 0.81989+0.7351logC
(mg/L)
20 mg/0.8 g
2.96 x 10-3
equation
t/q t =3.5233+
0.1022t
9.78
0.9892
1.2
e e
R2 = 0.9972

3.7. Isotherm study
The sorption data usually follow Langmuir [20] or Freundlich [21] isotherms models. The experimental data obtained for different initial fluoride concentration at constant temperature and pH was plotted (Figure 7a and7b from a Linearized form of Langmuir (7) and Freundlich (8) sorption isotherms equations stated as:
1.0
0.8
0.6
0.4
-0.6 -0.4 -0.2 0.0 0.2 0.4 0.6 0.8 1.0
logC


Ce = 1
qe Qo b

+ Ce
Qo
(5)
Fig.7. (b) Freundlich isotherm plot
log qe = log K f +
1

log Ce
n
(6)
It has clearly shown that the Freundlich model fitted the experimental data reasonably well (Fig.7b). The result of
the Freundlich isotherm model shows that the experimental
Where, q e is the amount of fluoride adsorbed per unit weight of the sorbent (mg/g), Qo is the sorption capacity for Langmuir isotherms (mg/g) and gives the maximum
data well fitted to the model with a correlation coefficient (R2=0.9972) indicating fluoride adsorption in the solution can be adsorbed on the mixed Al-Fe-Mn oxide adsorbent.
IJSER © 2014
http://www.ijser.org
International Journal Of Scientific & Engineering Research, Volume 5, Issue 3, March-2014
ISSN 2229-5518
890
3.8. Thermodynamic study
3.9 Effect of co-existing ions
2.1
2.0
1.9
1.8
1.7
1.6
1.5
1.4
1.3
lnK =7.6729-1861.12/T 90
R2 = 0.9897
88
87
86
72
0.0030 0.0031 0.0032 0.0033 0.0034
1/T(K-1)
Fig. 8. Plot of lnKc Vs T-1 for fluoride sorption on to amorphous adsorbent (pH = 6.02±0.01, dose 1.6 g/L, Co = 20 mg/L and contact time 3 h.
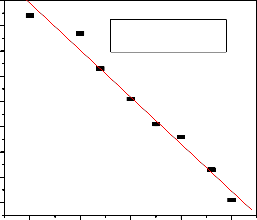
The sorption process is spontaneous in nature, as indicated by the negative value of ΔG0 = -3.52 KJ/mol (Fig.8). The negative value of ΔH0 = 16.57KJ/mol, suggests that the interaction of fluoride and Al-Fe-Mn mixed oxide is endothermic in nature. The positive value of ΔS0= (67.42KJ/mol), reflected that the reaction took place with increasing entropy; this is due to the increase in randomness with increase in number of species at the solid- liquid interface [8].

Table 3: Thermodynamic parameters for fluoride adsorption onto nano- sized adsorbent
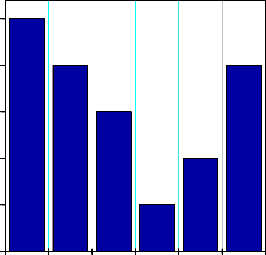
chloride nitrate sulfate phosphate carbonate all coexist.
coexsiting ions(20 mg/L)
Fig. 9. Effect of coexisting ions at pH = 6.02 ±0.01, contact time 3 h and dose 1.6 g/L
The effect of coexisting ions such as sulphate, chloride, phosphate, carbonate and nitrate on fluoride adsorption by the Al-Fe-Mn mixed oxide adsorbent were examined(Fig.9). Carbonates, chloride, sulfate and nitrate did not significantly interfere even with lower concentration 10 mg/L. However phosphate showed great significant competitive adsorption with fluoride [18].
T(K)
298
ΔG0 (KJ/mol)
-3.52
ΔH0 ( KJ/mol)
16.57
ΔS0 (J/molK)
67.42

IJSER © 2014
http://www.ijser.org
International Journal Of Scientific & Engineering Research, Volume 5, Issue 3, March-2014
ISSN 2229-5518
891
Table 4: Comparison efficency of various adsorbents for fluoride removal from water
Adsorbent | q e (mg/g) | Experimental conditions | Refer. |
Iron (III)- Tin(IV) (Mixed oxide) Fe-Al-Ce adsorbents Hydrous manganese Oxide coated alumina Hydrous Fe- Cr oxide Granular ferric Hydroxide (GFH) Hydrous Al- Fe-Mn mixed oxide | 10.47 178 7.09 31.88 7.0 23.99 | T=30oC, [F-]= 10 mg/L, pH =6.4 T=30oC, [F-] = 84.5 mg/L, pH =7.0 T=30oC, [F-] = 140 mg/L, pH =5.2 T=25oC, [F-]= 50 mg/L, pH =4-7.0 T=25 oC, [F-]= 100 mg/L , pH =6-7 T=25 oC, [F-]= 20 mg/L , pH =6-7 | [4] [25] [34] [18] [1] present work |
4. CONCLUSION
Ternary hydrous Al-Fe-Mn mixed oxide adsorbent was synthesized by co-precipitation method. Batch mode laboratory experiments were carried out to investigate adsorption kinetics and equilibrium. The surface structure of this oxide was investigated using SEM and XRD. The SEM image of the synthesized materials shows an amorphous structure. The adsorption process increases with the increasing the pH value until it reaches 6.02 and then it decreases. The equilibrium data were tested to fit Freundlich and Languimur models in order to understand the mechanism of fluoride adsorption at the surface of the sorbent. Thus, Freundlich equations were fitted more to the experimental data relative to Langmuir with (R2=0.9972) with maximum adsorption capacity of 23.99 mg/g. Adsorption kinetics followed and well described by a pseudo-second order model with correlation coefficients (R2=0.9892) for the present data. The thermodynamic studies revealed from the value of ΔG0, ΔH0 and ΔS0 of the adsorption of fluoride by hydrous Al-Fe-Mn mixed oxide
were an endothermic and spontaneous process with high degree of randomness. The presence of phosphate showed significant effect on removal efficiency of Al-Fe-Mn oxide adsorbent. Thus, amorphous phase Al-Fe-Mn mixed oxide has considerable potential for the removal of fluoride from aqueous solution.
Acknowledgements
The authors acknowledge sincerely their gratitude to the Department of Chemistry, Haramaya University, Ethiopia for the use of laboratory facilities.
References
[1] Kumar E.A.Bhatnagar, M.Ji, W.Jung, S.H.Lee, S.J.Kim, G.Lee, H.Song, J.S.Yang, B.H.Heon, 2009. Defluoridation from aqueous solution by granular ferric hydroxide. J.Water Res.43:,490-498.
[2] Amour R. and M.J.Basu, 2001. Adsorption of fluoride on to mixed metal oxide. Sep J.Purif.Tech. 24:121-127.
[3] Reimann C.K., B. Bjorvatn, Melaku Z., Teklehaimanot Reda., U.Siewersd, 2003. Drinking water quality in Eastern African Rift valley I- data and health aspects. Sci Tot.Environ.311:65-80.
[4] Biswas K., K.Gupta, U.C.Ghosh, 2009. Adsorption of fluoride by Hydrous iron (III)- tin(IV) bimetallic mixed oxide from queous solution.Chem.Eng.J.149:196-206.
[5] WHO, Fluorides Environmental health Criteria, World Health
Organization Geneva, Switzerland, 2004.pp 375-376.
[6] Chiony, N.J., 1991. Effects of fluoride on physiology of some animals and human beings. Indian environ.Toxicol., 1:17-32.
[7] Guo.X, G.Suna and Y.Sun, 2003. Oxidative stress from fluoride induced hepatotoxcity in rats. Flouride,36(1):25-29.
[8] Jamode,A.V., V.S.Sapkal, V.S.Jamode, 2004. Defluoridation of water using inexpensive adsorbent. J.Indian Ins.Sci.,74(5):163-171.
[9] Chaturvedi.A.K,. K.P.Yadava, K.C .Pathak, V.N.Singh, 1990.
Defluoridation of water by adsorption on fly ash. Water, Air, and
Soil pollution 49:51-60.
[10] Prarthasarathy N., J.Buffile and W.Haerdi, 1986. Combined use of calcium salts and polymeric aluminum hydroxide for deflouridation of waste water. Water Res 20(4):443-448.
[11] Ndiaye P.I.,P.Moulin, L.Dominguez, J.C. Millet, F.Charbit, 2005.
Removal of fluoride from electronic industrial effluents by membrane serration. Desalination,173(1):25-32.
[12] Schoeman J.and G.Botha, 1985. An evaluation of the activated alumina process for fluoride removal from drinking water and some factors influencing its performance. J.Water SA (1):25-31.
[13] Kumar E., A.Bhatnagar, M.Ji, W.Jung, S.H.Lee, S.J.Kim, G.Lee, H.Song, J.S.Yang, B.H.Heon, 2009. Defluoridation from aqueous solution by granular ferric hydroxide. J.Water Res.43:,490-498
[14] Beneberu Shemelis. Feleke Zewge and Chandravanshi B.S, 2006.
Removal of excess fluoride from water by aluminium hydroxide.
Bull.chem.Soc.Ethiop.20(1):17-34.
[15] Agrawal M., K.Rai, R.Shrivastav, S.Das, 2003. Defluoridation of water using amended clay. Cleaner production.11:439-444.
[16] Chaturvedi.A.K,. K.P.Yadava, K.C .Pathak, V.N.Singh, 1990.
Defluoridation of water by adsorption on fly ash. J.Water, Air, and
Soil pollution 49:51-60
[17] Shubo D., L.Han, Z.Wei, H.Jun, Y.Gang, 2011. Mn-Ce oxide as a high capacity adsorbents for fluoride removal from water. J.Hazard.186:1360-1366.
IJSER © 2014
http://www.ijser.org
International Journal Of Scientific & Engineering Research, Volume 5, Issue 3, March-2014
ISSN 2229-5518
[18] Biswas K., S.Debnath, U.C.Ghosh, 2010. Physicochemical aspects on fluoride adsorption for removal from water by hydrous iron (III)-Chromium (III) mixed oxide. Sep. Sci.Tech. 45:472-485.
[19] Jiao Z., Z. Zhang, Y.Yang, M.Huang, X.K.Ma, 2002.Removal of fluoride using rare earth based inorganic adsorbents. Environ.Chem.Chinese 21:365-370.
[20] Langmuir, I., 1918.The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical Society,
40(9): 1361–1403.
[21] Freundlich, H., 1906. Über die Adsorption in Lösungen.
Zeitschriftfürphysik.Chemie, 57, 385-470.
[22] McKay, G. and V. J. P.Poots, 1980. Kinetics and diffusion processes in color removal from effluent using wood as an adsorbent. J. Chem. Tech. Biotechnol. 30(1): 279–292.282.
[23] Sujana .M and S.Anand, 2010. Iron and aluminum based mixed hydroxides:A novel sorbents for fluoride removal form aqueous solution. J.applied surface science.256:6956-6962.
[24] Kloos H.and Tekle-Haimanot Reda, 1999. Distribution of fluoride and fluorosis in Ethiopia and prospects for control. J.Trop Med Int Health.4:355 –364.
[25] Zhang Y., X.Wu, X. Dou, M.Yang, 2007. Fluoride removal performance of novel Fe-Al-Ce trimetal oxide adsorbents. J.Chemosphere.69:1758-1764.
[26] Farrah H., J.Slavek and W.F.Pickering, 1987. Fluoride interaction with hydrous aluminum oxides and alumina. Aust.J.Soil Res.25:55-59.
[27] Nemade P.D., A.V.Rao., B.J.Alappat, 2002. Removal of fluorides from water using low cost adsorbents. J.Water-Science-and- Technology:-Water-Supply. 2(1):311-317.
[28] Onyango. M.S, Y.Kojima, O.Aoyi, E.C.Bernardo, H.Matsuda, 2004.
Adsorption equilibrium modeling and solution chemistry dependence of fluoride removal from water by trivalent-cation exchanged Zeolite F-9.J.Colloid.Interf.Sci 279,341-350.
[29] Puri B.K and S.Balani, 2000. Trace determination of fluoride using lanthanum hydroxide supported on alumina. J.Environ.Sci.Health part A.35:109-121.
[30] Reimann C.K., B. Bjorvatn, Melaku Z.,Teklehaimanot Reda.,U.Siewersd, 2003. Drinking water quality in Eastern African Rift valley I- data and health aspects. Sci Tot.Environ.311:65-80.
[31] Maliyekal,S.M; A.K Sharma; L. Phoilipet. J.Water res. 2006, 40;
3497-3506.
[32]Ho YS (2006) Review of second order models for adsorption systems. J Hazard Mater. 136:681–689.
[33] Lagergren S (1898) About the theory of so-called adsorption of soluble substances. K. Sven Vetenskapsakad Handl 24:1–39
[34] Shihabudheen M.;S.M Maliyekkal;K.Atul Sharma;P.Ligy,2006.
Manganese-oxide-coated alumina:A promising sorbent for defluoridation of water. J.water research 40:3497-3506.
[35] Cooney D.O., 1998. Adsorption design for waste water treatment, Lewis publisher, Boca Raton,USA.
892
IJSER © 2014
http://www.ijser.org










