International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 89
ISSN 2229-5518
Synthesis, characterization and catalytic activity studies of nano ZnO deposited on sintered calcium phosphate (ZnO/SCaP)
B.R. Saikishore kumar, S. Sumathi*, P.S. Raghavan*
Abstract-Nano ZnO was prepared by chemical methods on a novel solid support, namely, calcium phosphate .Different loading levels of nano zinc ox- ide over the support were prepared and influence of temperature on the synthesis was studied. The catalysts were characterized by physico-chemical methods. The XRD and UV-DRS showed formation of ZnO and high resolution SEM image showed formation uniform nano particles. The catalyst was found to be efficient in Knoevenagel condensation when compared with unsupported analogue.
Key words: Nano ZnO; SCaP support; Knoevenagel condensation, Coumarins, Bulk zinc oxide; Chemical deposition of ZnO; UV-DRS of ZnO.
1 INTRODUCTION
—————————— ——————————
Zinc oxide has been reported to be an efficient catalyst in many organic reactions [1]. The structural changes at the nano level greatly influence the reaction rates [2, 3]. A large number of reports are available on the utility of nano zinc oxide [4, 5] in catalyzing organic reactions. In the design of a catalyst, the catalyst-support plays an important role for fixing the active- centre firmly, providing large surface area and imparting acid- ity to the catalyst. Conventionally, silica, alumina, zeolites, clay, etc., are used as catalyst-support. In the present investi- gation sintered calcium phosphate (SCaP) [6] was used as cata- lyst-support. The SCaP has P-O-P chains containing terminal P-O-H groups and cross-linked by calcium ions [7] and hence, it is expected to be acidic in nature.
2 EXPERIMENTAL
2.1 Preparation of catalyst-support (SCaP)
It involves forming a paste of calcium carbonate (pre- cipitated 99%) using distilled water. To this paste, phosphoric acid was added drop-wise under continuous stirring. The re- action slurry was transferred to alumina crucible and dried in hot air oven at 150°C for 8 h. The dried content was sintered at
750°C for 30 minutes in a static air atmosphere using muffle
furnace [6]. The sintered mass was then crushed to powder using mortar pestle and sieved through 325 mesh .The sieved powder was used as a catalyst-support for depositing ZnO..
————————————————
• B.R.Saikishore kumar is currently pursuing research program at Hindustan University, Chennai, India, and PH-044 27474262. E-mail: saikishore.482@gmail.com
• S. Sumathi and P.S. Raghavan are currently doing research in organic
chemistry and materialscience in Hindustan University, Chennai, India,
and PH-044 27474262. E-mail: sumathi@hindustanuniv.ac.in, &
raghavan@hindustanuniv.ac.in
2.2 Characterization
Powder X-ray diffraction was recorded by using GE Analytical XRD (Model: XRD 3003 T/T) 2θ value ranging from
10-70 degree. The SEM analysis was carried out using a Quanta Field Emission SEM (Quanta-200F). UV-visible spectra were recorded using Perkin Elmer spectrometer (LAMBDA-
850 UV-Visible Spectrophotometer) in reflectance mode F.
2.3 Reaction
To a 50 ml round bottomed flask, calculated quanti- ties of salicylaldehyde, ethylacetoacetate & ZnO/SCaP were introduced. It was fitted with double-walled condenser and cold water was circulated. The entire reaction set up was placed in oil bath and heated to desired temperature under continuous stirring using magnetic stirrer. The progress of reaction was monitored by TLC and the product distribution was analysed using HPLC.
.
3 RESULTS & DISCUSSION
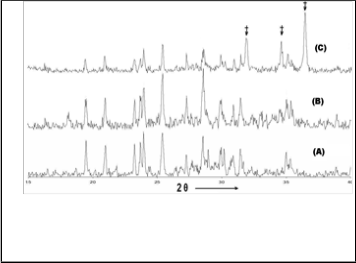
In the preparation of the catalyst, the zinc salt is precipitat- ed as zinc hydroxide using the ammonia solution. If excess ammonia was added, the precipitate dissolves forming zinc complex. The volume of water used, the stirring speed and rate of addition of ammonia plays vital role in deciding the particle size of ZnO. The zinc hydroxide gets converted to zinc oxide at 125°C. Hence, 2 different temperatures were used for calcining the catalyst, viz., 150°C and 400°C. The XRD pattern of ZnO/SCaP (400) completely matches well with ZnO phase [8] (Figure 1) (PDF No. 891397) while, ZnO/SCaP (150) did not show formation of ZnO. This may be due to strong binding between zinc species on the support, which would have increased the decomposition temperature of zinc hydroxide.
IJSER © 2015
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 90
ISSN 2229-5518
The SEM images also supported the XRD observation. The ZnO/SCaP calcined at 400°C showed formation of nano particles of ZnO with uniform particle size distribution in the range of 20 to 30 nm (Figure 3) while, that calcined at 150°C showed agglomerates without distinct particles (Figure 4). Hence, the calcining temperature of the catalyst was opti- mized as 400°C.
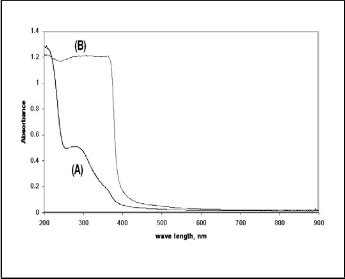
The UV-DRS spectra of ZnO/SCaP (400) showed a sharp
absorption at around 380 nm. Dhakshinamoorthy et.al, [9] have studied absorption behaviour of bulk ZnO and nano ZnO. The bulk ZnO absorbed at around 300 nm while that of nano ZnO absorption between 380-400 nm. This observation was completely matching with the present study, where ZnO/SCaP (150) absorbed at around 300 nm corresponding to bulk ZnO and that calcined at 400°C showed absorption at around 400 nm corresponding to nano ZnO.
As a preliminary investigation evaluation of catalytic ac- tivity was carried out by taking 1.22 g of salicylaldehyde (1 eq.), 1.62 g of ethylaceto acetate (1.25 eq.), and 0.324 g of cata- lyst. This reaction mixture was subjected to heating at 120°C [10], under continuous stirring. The progress of the reaction was monitored by TLC (2% ethyl acetate & hexane). The in- fluence of time was studied using different catalyst and the results are presented in Table 1. The conversion increases with reaction time and reaches 100% within 2 hours, whereas over bulk ZnO as a catalyst requires 10 hours for complete conver- sion. Reaction with blank catalyst-support (SCaP) showed 0% conversion.
The catalyst calcined at 150°C showed very low activity whereas the unsupported ZnO requires 8 hours for complete conversion .Thus, the ZnO/SCaP (400) was found to be superior in this condensation, in comparison with bulk ZnO.
TABLE 1
Knoevanagel condensation over ZnO/SCaP catalysts.
Figure 1: XRD patterns of (A) SCaP-750 (blank), (B) ZnO/SCaP(150) and

(C) ZnO/SCaP(400)
Figure 2: SEM image of ZnO/SCaP(400)

. Figure 3: SEM image of ZnO/SCaP(150)
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 91
ISSN 2229-5518
[8] Schulz, H., Thiemann, K.H.,”Structure prediction and energy land- scape exploration in the zincoxide system”, Solid State Commun. 32,
783, 1979.
[9] Amarajothi Dhakshinamoorthy, Pitchai Visuvamithiran, Vairape- rumal Tharmaraj, Kasi Pitchumani, “Clay encapsulated ZnO nano- particles as efficient catalysts for N-benzylation of amines”, Catalysis Communications, 16 15-19, 2011.
[10] B.Vinaykumar,” ZnO nanoparticle as catalyst for efficient green one- pot synthesisof coumarins through Knoevenagel condensation”, J.Chem. Sci., 123, 615-621,2011.
Figure 4: UV-DRS spectra of (A) ZnO/SCaP(150) and (B) ZnO/SCaP(400)
4 CONCLUSION
Nano ZnO was prepared over non-conventional support, namely sintered calcium phosphate. Influence of temperature during synthesis and physico-chemical characterization proved that ZnO/SCaP contains nano particles of zinc oxide. The catalyst was found to be efficient in Knovenagal condension with 100% conversion within 3 hours.
ACKNOWLEDGMENT
The first author thanks Hindustan University for providing research fellowship and CENCON, Sathyabhama University and B.S.Abdur Rehaman University for characterization.
REFERENCES
[1] Mona Hosseini-sarvari,” Green solvent –free reactions on zno”, Green Chemistry Environmentally Benign Approaches. Edited by Dr. Mazaahur Kidwai, 2012.
[2] Utkarsha U Indulkar,” Ecofriendly and facile Nano ZnO catalyzed solvent-free enamination of1,3-dicarbonyls“,Tetrahedron letters, 53,
3857-3860, 2012.
[3] F.Matloubi Moghaddam,” Rapid and eff ic ient one-pot synthesis of 1,4-dihydr opyr idine and polyhydroquinoline der ivatives through the Hantzsch f our compone nt condensation by z inc oxide”,J. Iran. Chem. Soc., 6, 317, 2009.
[4] David Ian Magee,”Highly efficient one-pot three-component Mannich reaction catalyzed by ZnO-nanoparticles in water156” General papers, ARKIVOC 9 156, 2011.
[5] Nay Barry “Zinc oxide composition for use in catalysis”, US Pat.5811365, 1998.
[6] S.Induja, P.S.Raghavan,”Catalytic efficiency of CuO in degradation of phenol using sintered calcium phosphate (SCaP) as a catalyst support “,Catalysis Communications, 33, 7-10, 2013,
[7] Sidney Omelon, Andrew Baer, Tom Coyle, Robert M Piiliar,” Pol- ymeric crystallization and condensation of calcium polyphos- phate glass” Mater. Res. Bull., 43, 68-80, 2008.
IJSER © 2015 http://www.ijser.org



