International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1056
ISSN 2229-5518
Synthesis and Spectroscopic studies on Manganese(II), Copper(II), Nickel(II), and Cobalt(II) Complexes derived from L-Valinol and pyridine aldehyde and their Antimicrobial studies
Netra Pal Singh*, Gauravb and Jagvir Singhc
aDepartment of Chemistry, Meerut College(CCS University), Meerut 250001, U.P. India
b Mewar University, NH-79, Gangrar , Chhitorgarh, Rajasthan
c Department of chemistry, ARSD College, Dhaula Kuan, New Delhi
* Email : npsmcm.in@gmail.com
Abstract- Novel metal complexes of the type[M(L)2 ]( where M=Mn(II), Cu(II), Ni(II),
and characterized by elemental analysis, molar conductance , magnetic moments, FTIR,, 1H
Co(II); L= Schiff base derived from L-valinol and pyridine aldehyde) have been synthesized
and 13C NMR and UV-visible techniques. On the basis of spectral studies, octahedral geometry has been assigned for all metal complexes. In vitro antimicrobial activity of ligand and metal complexes were also studied against bacteria (Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Salmona typhi) and fungi (Candia albicans and Candida parappsilosis) which show antimicrobial activity of ligand and metal complexes. Metal complexes showmore activity against bacteria and fungi as compare to pure ligand
Key Words: Schiff base, metal complexes, spectral studies, antifungal, antibacterial
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1057
ISSN 2229-5518
1 INTRODUCTION
Metallo-drugs are becoming an interesting research area after the discovery of cisplatin[1]. Since then, many complexes have been synthesized and tested on a number of biological systems. New areas of research, which mainly focused on the specific synthesis, highly functional metal based drug complexes, have drawn considerable attention. Schiff bases and their complexes have a variety of applications in biological, clinical, analytical and pharmacological areas[2]. When ligands coordinate to transition metals, it is believed that the selectivity towards certain biological systems is improved[3-5]. The enormous interest in the field of coordination chemistry of transition metal ions with Schiff bases is due to use of
researchers in
Ithe fiJeld of coSordination cheEmistry of thRese metal ions[7]. N and O
these compounds as biological models, as oxygen carriers and as drugs[6]. Recently
synthesized Cu(II), Ni(II), Co(II), Zn(II) and Fe(III) complexes show current interest of
coordinating ligand and their transition metal complexes have been found to possess important catalytic as well as biological activity[8]. Some other transition metal complexes have also been synthesized recently which show good biological activities viz. antimicrobial, toxicity, fluorescence quenching, DNA interaction and antitumor[9-14].In this article, we synthesized novel Schiff base and their metal complexes and were characterized by IR, NMR, Molar conductance, and Magnetic moments, elemental analysis, mass spectrum and UV-visible spectroscopy.
2 EXPERIMENTAL
All chemicals were of A.R. grade. MeOH, EtOH, diethyl ether and metal salts were purchased from Qualigens. 2-pyridine aldehyde and L-Valinol were purchased from (Sigma
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1058
ISSN 2229-5518
Aldrich). All chemicals were used as obtained without further purification. Elemental analysis (C, H, N) were performed using a Carlo Erba 1106 elemental analyzer. Metals and chlorides were determined volumetricall[15] and gravimetrically[16] respectively. IR spectra were recorded using KBr discs (4000-400 cm-1) on a Shimadzu 8300 IR spectrophotometer.
Electronic absorption spectra in the 200-900 nm range were obtained in DMF (10-4 M) on a
Systronic UV-visible spectrophotometer. Molar conductance measurements were determined in DMSO (~10-3 M) at room temperature using a Jenway Model 4070 conductivity meter. Magnetic moment measurements were carried out by the Gouy method using Hg[Co(SCN)4 ] as calibrant. 1H and 13C NMR spectra (at room temperature) (in DMSO-d6 , ~10-3 M) were recorded on a Bruker AC 700L NMR spectrometer with reference to TMS (tetra methyl silane). Chemical shifts were reported on the δ scale. ESR spectra of Cu(II) complex was recorded as polycrystalline sample in acetonitrile solution, at room temperature on a E-112
2.1 Synthesis of ligand N-(2-Hydroxyethyl)-pyridine-2-aldimine
ESR spectrophotometer employing DPPH as the g-marker.
A mixture of pyridine-2- aldehyde and L-Valinol in 1:1 molar proportion in an alcoholic medium containing a few drops of concentrated HCl was refluxed for 3h. The product was
separated , filtered, washed with alcohol and recrystallized from EtOH (Fig 1).
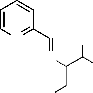
N CH3
N
CH3
HO
Fig 1. 3-Methyl-2-[(pyridin-2-ylmethylene)-amino]-butan-1-ol
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1059
ISSN 2229-5518
Analytical data of 3-Methyl-2-[(pyridin-2-ylmethylene)-amino]-butan-1-ol
Ligand (C22 H28 N4 O2 ): Yield: 52%; M.P. 230 0C, Mol. wt. 382, color: yellowish; analytical data for LIGAND found (calc.): C, 69.10 (69.24); H, 7.85 (7.92); N, 7.32 (7.43). IR (KBr, cm-1): 3216 ν NH , 1640 νC=N , 3585 νOH , 2228 νC-N . ESI-MS, m/z Data found (calc.): 382.01 (381.11), 1H NMR (DMSO-d6 ) δ ppm: 10.4 (s, CH=N), 3.40 (s, CH2 ),
3.29(s,Pyr.H),2.70(br,1 H, OH), 13C NMR (DMSO-d6 ) δ ppm: 161.4 ( CH=N), 65.6
(OCH2 ), 189.8(Pyri C).
2.1 Synthesis of Metal Complexes
An alcoholic soIlutionJof Schiff Sbase (2mmol)Ewas refluxedRwith 1mmol of CoCl2 .6H2 O/
NiCl2 .6H2 O/ CuCl2 .2H2 O/MnCl2 .6H2 O in ethanol on water bath for 2 h; few drops of
sodium acetate was added and refluxed for 4h. The separated complex was filtered, washed thoroughly with water, ethanol and ether and finally dried in vacuum over fused CaCl2 (Fig2.).
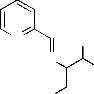
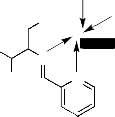
H3C
N
O N M
N O CH3 N
CH3
CH3
(Where M = Mn(II), Co(II), Ni(II) and Cu(II)
Fig 2. Structure of metal complexes
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1060
ISSN 2229-5518
Analytical data of Metal Complexes
Complex 1. (C22 H28 CuN4 O2 ): Yield: 50%; M.P. 290 0C, Mol. wt. 443.54, color: blueish; found (calc.): C, 59.52 (59.61); H, 6.31 (6.43); N, 12.62 (12.69), Cu, 14.37(14.48). IR (KBr, cm-1): 3216 ν NH , 1640 νC=N , 2228 νC-N , 465νCu-N , 519νCu-O, . ESI-MS, m/z Data found (calc.): 443.54 (442.55), 1H NMR (DMSO-d6 ) δ ppm: 10.4 (s, CH=N), 3.40 (s, CH2 ),
3.29(s,Pyr.H), 13C NMR (DMSO-d6 ) δ ppm: 161.4 ( CH=N), 65.6 (OCH2 ), 189.8(Pyri C)
Complex 2. (C22 H28 NiN4 O2 ): Yield: 50%; M.P. 290 0C, Mol. wt. 438.69, color: light blue; found (calc.): C, 60.17 (60.40); H, 6.38 (6.49); N, 12.76 (12.70), Ni, 13.37(13.45). IR (KBr, cm-1): 3216 ν NH , 1640 νC=N , 2228 νC-N, 467νNi-N , 518νNi-O, .. ESI-MS, m/z Data found (calc.): 438.69 (437.69), 1H NMR (DMSO-d6 ) δ ppm: 10.4 (s, CH=N), 3.40 (s, CH2 ),
3.29(s,Pyr.H), 13C NMR (DMSO-d6 ) δ ppm: 161.4 ( CH=N), 65.6 (OCH2 ), 189.8(Pyri C).
found (calc.): C, 60.69 (60.71); H, 6.43 (6.55); N, 12.87 (12.88), Mn, 12.62(12.69). IR
Complex 3. (C22 H28 MnN4 O2 ): Yield: 45%; M.P. 305 0C, Mol. wt. 434.93, color: Pinkish;
(KBr, cm-1): 3216 ν NH , 1640 νC=N , 2228 νC-N, 467νMn-N , 519νMn-O,.. ESI-MS, m/z Data found (calc.): 434.93 (433.83), 1H NMR (DMSO-d6 ) δ ppm: 10.4 (s, CH=N), 3.40 (s, CH2 ), 3.29(s,Pyr.H), 13C NMR (DMSO-d6 ) δ ppm: 161.4 ( CH=N), 65.6 (OCH2 ),
189.8(Pyri C).
Complex 4. (C22 H28 CoN4 O2 ): Yield: 55%; M.P. 298 0C, Mol. wt. 438.93, color: pinkish; found (calc.): C, 60.14 (60.80); H, 6.37 (6.40); N,. 12.75(12.79), Co, 13.42(13.52). IR (KBr, cm-1): 3216 ν NH , 1640 νC=N , 2228 νC-N, 464νCo-N , 520 νCo-O,.. ESI-MS, m/z Data found (calc.): 438.93 (437.93), 1H NMR (DMSO-d6 ) δ ppm: 10.4 (s, CH=N), 3.40 (s, CH2 ), 3.29(s,Pyr.H), 13C NMR (DMSO-d6 ) δ ppm: 161.4 ( CH=N), 65.6 (OCH2 ),
189.8(Pyri C).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1061
ISSN 2229-5518
3 In Vitro Antibacterial and Antifungal Studies
Antibacterial and antifungal study of newly synthesized ligand (L) and all metal complexes were done in vitro by reported method[15]. The stock solution (1 mg ml-1) of the test chemical was prepared by dissolving 10 mg of the test chemical in 10 ml of DMSO. The stock solution was suitably diluted with sterilized distilled water to get 500 and 100 µg ml-1. Control for each dilution was prepared by diluting 10 ml of solvent instead of stock solution with sterilized distilled water. All compounds were evaluated for their in vitro antibacterial activity against Bacillus subtilis and Escherichia coli and antifungal activity against Aspirgillus niger and Aspirgillus flavus by the agar-well diffusion method. Bacteria were
inoculated into NIutrienJt Broth (DSifco) and incubEated for 30 h Rand the fungi studied incubated
in Malt Extract Broth (Difco) for 54 h. In the agar-well diffusion method, Mueller Hinton
Agar (Oxoid) for bacteria and Malt Extract Broth (Difco) sterilized in a flask and cooled to ~
48 °C was distributed (20 ml) to sterilized petri dishes after injecting 0.01 ml culture of bacterium prepared as mentioned above and allowed to solidify. The dilution plate method was used to enumerate microorganism (105 bacteria per ml and fungi 103-104 per ml) for 24 h. By using a sterilize cork borer (7 mm diameter), wells were dug in the culture plates. Compounds dissolved in DMSO were added (0.2 µ) to these wells. The petri dishes were left at 4 °C for 2 h and then the plates were incubated at 30 °C for bacteria (24-28 h) and at 25 °C for fungi (78 h). At the end of the period, inhibition zones formed on the medium were evaluated as millimeters (mm) diameter. Biological activity data of all compounds were expressed as inhibition level % over control calculated from the size of inhibition zone. The percent inhibition was calculated using the formula:
Inhibition level, % = (C – T)100 / C
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1062
ISSN 2229-5518
Where C is the diameter of the microbial colony in the control plate and T is the diameter of the microbial colony in the tested plate after same incubation period.
4 RESULTS AND DISCUSSION
The newly synthesized ligand and its metal complexes are stable at room temperature in solid state. The ligand is soluble in common organic solvents but metal complexes are soluble in DMF and DMSO. The analytical data are in good agreement with the proposed stoichiometry of the complexes.
4.1 IR Spectral Studies
The IR spectra of ligand shows a sharp band at 3585 cm-1 and 1576 cm-1 which show the
complexes, no υ(OH) vibration was found and υ(C=N) are shifted by 1598-1626 cm-1
presence of of υ(OH) and υ(C=N) vibrations respectively. In the IR spectra of metal
which shows the coordination of (C=N)[ 17]. A new band appeared in the range 460-466 cm-
1 and 515- 530 cm-1may be assigned υ(M-N) and υ(M-O) vibrations respectively[18].
4.2 Electronic Spectra
In the spectrums of UV-Vis spectroscopy, the electronic spectra of Mn(II) complexes show two bands in the regions 22500 - 22650 and 18500 - 18850 cm-1 which may be assigned to 6A1g
→4T2g
and 6A1g
→ 4T1g (G) transitions respectively suggesting octahedral environment around
the Mn(II) ion29 . The magnetic moment 4.88 BM is an additional evidence for an octahedral structure. The electronic spectra of the cobalt (II) complex showed three bands at 8780-8810,
17475-17775 and 30235-30270 cm-1, which may be assigned to 4T1g → 4T2g (F), 4T1g →
4T1g(P), and 4T1g → 3A2g (F) transitions, respectively, and suggested octahedral geometry
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1063
ISSN 2229-5518
around the cobalt ion.. The electronic spectra of the copper(II) complex display a broad band at 14920 cm-1 due to 2B1g → 2Eg and two bands at 16390 and 27250 cm-1 assigned to d–d transitions and a charge transfer band respectively, of an octahedral environment. The
moments in such complex, as in apparent, lie appreciably above the spine-only value (1.73
BM), but as the electronic ground states are non-degenerate this cannot arise from inherent angular momentum in the ground state. It arises due to mixing in of some orbital angular momentum from excited states via spin orbit coupling. The copper (II) complex exhibit magnetic moments of 1.78 B.M., respectively, at room temperature. These values are quite close to the spin-allowed values expected for an S =1/2 system and may be indicative of a distorted octahedral geometry around copper (II) ion. The nickel(II) complex was insoluble in common organic solvents and water. The compound was soluble in coordinating solvents,
sphere. The nickel(II) complex exhibited three bands at 9960-10165, 15850-16155, and
pyridine and DMSO, presumably by displacing water molecules from the coordination
29940-29985 cm-1 assignable, respectively to the transitions 3A2g(F)→ 3T2g(F) (ν1) , 3A2g(F)
→ 3T1g(F) (ν2 ) and 3A2g (F) → 3T2g (P) (ν3 ), which are characteristic of nickel(II) in octahedral geometry. Magnetic behavior of octahedral nickel(II) complex is relatively simple. From both the simple ‘d’ orbital splitting diagram and the energy level diagram, all the octahedral complexes of the divalent nickel should have two unpaired electrons. The experimental magnetic moment values usually lie in rang 2.9 to 3.4 BM, a little higher than spin only value (2.83). The electronic spectra of the cobalt (II) complex showed three bands at 8780-8810, 17475-17775 and 30235-30270 cm-1, which may be assigned to 4T1g → 4T2g (F), 4T1g → 4T1g (P), and 4T1g → 3A2g (F) transitions, respectively, and suggested octahedral geometry around the cobalt ion. The cobalt(II) complex shows magnetic moment values[18] of 4.85 B.M. at room temperature. This high value of the magnetic moments and the
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1064
ISSN 2229-5518
stoichiometries suggest a coordination number of six for the central cobalt(II) ion and an octahedral geometry.
4.3 1H and 13C NMR Spectra
1H- NMR and 13C-NMR of ligand in DMSO-d6 solution show that they are NMR active. The 1H-NMR spectrum of free ligand show a singlet at 10.4 ppm due to imine proton , 3.40 ppm due to CH2 proton, 3.29 ppm due to pyridine proton respectively. 13C-NMR spectrum of free ligand show at 161.4 ppm in the range due to imine carbon ,65.6 ppm due to OCH2 and
189.8 ppm due to pyridine carbon. Thus, 1H- and 13C- NMR spectral data support proposed structure of ligand and metal complexex and as well coordination behavior of ligand.
4.4 Molar Conductance Measurement
The molar conductance (⋀M) values of all metal complexes were measured in DMSO and
non electrolyte nature[19].
4.5 ESR spectrum of Cu(II) metal complex
The ESR spectrum of Cu(II) complex shows g║ and g┴ values are 2.02 and 2.16 with g av calculated to be 2.11. The Cu(II) complex, shows a compressed octahedron with g║ < g ┴ indicating the electron is delocalized in the dz2. The parameter G, determined as G =( g║ -2/ g┴ - 2) is.126 suggesting considerable interaction in the solid state.
4.6 In vitro antimicrobial activity
In vitro antimicrobial activity of newly synthesized ligand and metal complexes have been tested against the bacteria Bacillus subtilis and Escherichia coli and fungi Aspirgillus niger
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1065
ISSN 2229-5518
and Aspirgillus flavus and are summarized in Table1.
Table 1. Antibacterial and antifungal activity data of ligand and metal complexes (%
inhibition)

Compound *Conc. a b C d

L 100
500
40 51 62 65
48 58 74 78
[Cu(L)2 ] 100
500
[Co(L)2 ] 100
48 54 71 72
53 55 83 85
| 500 | 61 | 82 | 89 | 86 |
[Ni(L)2] | 100 | 46 | 59 | 69 | 70 |
| 500 | 52 | 64 | 77 | 79 |
[Mn(L)2 ] | 100 | 51 | 70 | 81 | 78 |
| 500 | 61 | 82 | 89 | 86 |

* = (𝛍g ml-1), a = Bacillus subtilis, b = Escherichia coli, c = Aspirgillus niger , d = Aspirgillus flavus.
The values indicate that all complexes have higher antimicrobial activity than the free ligand. Among all tested metal complexes Co(II) metal complex show highest biological activity
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1066
ISSN 2229-5518
against all microbes. Such increased activity of the metal chelates can be explained on the basis of chelation theory. On chelation, the polarity of the metal ion will be reduced to a greater extent due to overlap of the ligand orbital and partial sharing of the positive charge of the metal ion with donor groups. Further, it increases the delocalization of π-electrons over the whole chelate ring and enhance the penetration of the complexes into lipid membranes and blocking of the metal binding sites in enzymes of microorganism. These complexes also disturb the respiration process of the cell and thus block the synthesis of proteins, which restricts further growth of microorganism[20].
5 Conclusions
Newly synthesized Ligand and all the metal complexes have been characterized by analytical and spectral studies. Octahedral geometry and non electrolyte nature has been assigned to all metal complexes. Biological investigations show that metal complexes are more biologically
active than ligand.
6 Acknowledgements: The authors are thankful to ACBR, Delhi for providing spectral data, IIT- Delhi, for NMR, CDRI, Lucknow for providing elemental analysis data and SARC, Meerut for biological activity. Authors are also thankful to Principal and Head of Chemistry department, Meerut College, Meerut for providing lab facility.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1067
ISSN 2229-5518
REFERENCES
1. C. Orvig and M.J. Abrams, “Medicinal Inorganic chemistry: Introduction” Chem. Rev., vol.
99, pp. 2201-2204, 1999
2. A. Banerjee, A. Guha, J. Adhikary, K. Manna, s. Dey,E. Zangrando and D. Das, “Dinuclear Co(II) complexes of Schiff base compartmental ligands :Synthesis, crystal structure and biorelevant catalytic activities” , Polyhedron, vol.60, pp.102-109, 2013
3. A. Tavman, N. M. Agh-Atabay, S. Güner, F. Gücin and B. Dülger, “Investigation of Raman, FTIR, EPR spectra antibacterial activity of 2-(5-H/Me/Cl -1-H-benzimidazol-2-yl) phenol ligand and their Fe(NO3 )3 complexes” Transition Met. Chem. ,vol.32, pp.172-179, 2007
4. A. Tavman, N. M. Agh-Atabay, A. Neshat, F. Gücin, B. Dülger and
2-yl)4-bromonitrophenolligands and Fe(NO3 )3 complexes” Transition Met. Chem. vol.,31,
D.Haciu,”Structuralcharacerization and antimicrobial activity of (5-H/methyl)1hbezimidazol-
pp. 194-200, 2006
5. Q. Zhou and P. Yang, “Crystal Structure and DNA binding studies of a new Cu(II) complex involving benzimidazole”, Inorg. Chim. Acta., vol. 359,pp. 1200-1206, 2006
6. T. Ozawa and A.Hanake, “ESR evidence of the formation of a new superoxide complex of
tetra-p-tolylporphyrinato Co(II) in aprotic solvents”Inorg. Chim. Ata.,vol. 151, pp. 201-204,
1988
7. S.K.Padhi, R.Sahu and V. Manivannam,”Synthesis and structure of cobalt(II) alcoholate complexes formed by addition of a water molecule across 2-pyridylsubstituted imine function” Inorg. Chim. Acta, vol. 367,pp.57-63, 2011
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1068
ISSN 2229-5518
8. R. K. O. Sigel, B. Song and H. Sigel, Stabilities and stcture of metal on complexes of adenosine5’-Othiomono phosphate in comparison withthose of its parents nucleotide in aquous solution” J. Am. Chem. Soc.,vol. 119, pp. 744-755, 1997
9. S. Tabassum, N. P. Singh, and F. Arjmand, “Synthesis, Characterization and Toxicity of Heterobinuclear Complexes of Transition Metal ions” Synth. React. Inorg. Met.Org. Chem.,vol. 31, no.10, pp. 1803-1815, 2001
10. S. Tabassum, N. P. Singh and J. Mussarat, “Synthesis and Characterization of the Copper (II) Complex with 2, 2-bis (1H, 3H, 5H) Pyrimidine-4, 6-dione-1, 2 diiminoethane: Flourescnece Quenching Studies in Proteins” Synth. React. Inorg. Met.Org. Chem., vol.33, no.3,pp. 509-
517 ,2003
no.7, pp. 917-92I7, 201J0
SER
11. K. Singh and D. Pal, “Synthetic structural and biological studies of Organosilicon (IV)
complexes of Schiff base derived from pyrole2-2carboxaldehyde” J. Serb. Chem. Soc.,vol.75,
12. S. Chandra, M. Tyagi and S. Agarwal,”Synthesis, characterization of tetraazamacrocyclic ligand and its Co(II), Ni(II) and Cu(II) complexes. J. Serb. Chem. Soc.,vol.75, no.7,pp. 935-
941, 2010
13. N. P. Singh and A. N. Srivastava, “Synthesis, Characterization and Spectral studies of heterobinuclear complexes of transition metal ions and their biological activity”
Int. J. of Chem. Env. And Pharm. Research, vol. 1,no.1,pp. 27-31, 2010
14. N. P. Singh and A. N. Srivastava, “Synthesis, Characterization and Spectral studies of Novel heterobinuclear complexes of transition metal ions and their biological activity” E-J.
Chem., vol.8,no.2, pp.809-814, 2010
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1069
ISSN 2229-5518
15. R. C. Maurya, J. Chourasia and P. Sharma, “Bis(O-vaniline)benzidine (O-V2 BzH2 )as a binucleating ligand :Synthesis, characterization and 3D molecule modeling and analysis of O- V2 bzH2 with Cu(II), Ni(II), Co(II), Mn(II), Zn(II)(,Sm(II) and dioxournium”, Indian J. Chem., vol.47(A), pp.517-528, 2008
16. M. Sonmez and M. Sekerci, “Synthesis, characterization of Cu(II), Co(II), Ni(II) and Zn(II) Schiff base complexes from 1-amino-5- benzoyl-4-phenyl- 1H- pyrimidine -2-one with salicyaldehyde”, Polish J. Chem.,vol. 76,pp. 907-914, 2002
17. M. Tumer, N. Deligonul, A. Golcu, E. Akgum, M. Dolaz, H. Demirelli and M. Digrak, “Mixed ligand copper(II) complexes investigation of their spectroscopic catalysis, antimicrobial and potentiomeric properties”, Transition Met. Chem.,vol. 31, pp. 1-12, 2006
18. J. Pons, A. Chadghan, J. Casabo, A. Alvarez-Larena, J. F. Piniella, and J. Ros, “Copper(II)
pridl)pyrazole)copper(II) nitrate” Polyhyedron, vol. 20 ,pp. 2531-2536, 2001
complexes with pyrazole derived ligands; crystal structure of (diaquanitrato(phenyl-5(2-
19. W. J. Geary, “Molar conductance”,Coord. Chem. Rev.,vol. 7, pp.81-121,1971
20. M.Montazerozohai, S.Yadegari and A. Nagiha , “Synthesis , characterization , electrochemical behavior and antibacterial/ antifungal activities f[Cd(L)X2] complexes of Schiff base ligand”, J. Serb.Chem. Soc.,vol. 78, pp.1-18, 2013
IJSER © 2013 http://www.ijser.org


