International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 28
ISSN 2229-5518
Synthesis and Characterisation of Some New
Organoantimony (V) Aryloxyacetates
Kiran Singhal, Dharmendra K. Srivastava, Prem Raj, A. Pandey
Abstract— A series of new tri and tetra-organoantimony (V) aryloxyacetates of the general formula R3 Sb(OCOCH 2 OR’) 2 ; and R4 Sb(OCOCH2 OR’) [where R = p-OCH 3 C 6 H4 -, C 6 H5 - & R’ = p-CH3 C6 H4 -, m-CH 3 C6 H 4 -, o-CH3 C 6 H4 -, β-C10 H 7 -, o-ClC6 H 4 -], have been synthesised and characterised. These complexes are monomeric in benzene and non-electrolyte in acetonitrile. IR, NMR spectral data suggest the hepta-coordination with weak secondary interaction in between antimony and carbonyl oxygen of aryloxyacetate ligands.
Keywords— Tri and tetra-organoantimony (V) aryloxyacetates, IR, 1H NMR, 13CNMR spectra, monomeric, non ionic, secondary interaction.
—————————— ——————————
The great variety of structural possibilities offered by organo- metal carboxylates perhaps is the main factor for the contin- ued interest in this class of compounds [27-34]. Despite a con- siderable interest in organometallic carboxylates and the varie- ty of metals for which such derivatives have been synthesized, corresponding aryl oxyacetates are mainly confined to tin. The etheral oxygen may or may not involve in coordination with metal atom and thus play an important role in changing the geometry of the complex. Studies on organotin complexes of aryloxy acetic acids indicate intramolecular O-Sn coordination particularly in case of alkyl tin derivatives with monomeric constitution in solid state. In sharp contrast to this triphenyltin derivatives having pentacoordination around tin were found to be polymeric with bridging carbonyl group. A perusal of literature reveals that the role of pentacoordination, partucu- larly in case of group 14 and group 15 elements in reaction mechanism is potentially one of the best method to explore the possibility of conformation extended from the ideal trigonal bipyramidal to the square or rectangular bipyramidal geome- try and the compounds of pentacoordinated phosphorus, ar- senic and antimony are probably the most interesting [1-10].
In case of thioacetate secondary bonding takes place through
chelation [2, 6, 7 ]. Another important group of ligand which has not attracted much attention, but closely resembles to car- boxylates, is aryl oxy acetates. However unlike acetate or thio- acetate, aryl oxy acetates has one extra donor site i.e.; ArO, in addition to the C = O functionality.
It is note worthy that despite such a great variety of structural possibility, as discussed above, exhibit by organom
————————————————
• Corresponding author is Dr. (Mrs.) Kiran Singhal, Associate Professor, Chemistry Department, University of Lucknow, Lucknow and is the thesis supervisor of Mr. Dharmendra K. Srivastava.
• Email: singhal.kiran@gmail.com Ph: +91-9415159894
• Contract Grant Sponsor: University Grants Commission, New Delhi,
India Vide Letter No. 37-429/2009 SR.
• Dr. Prem Raj is Senior Professor Chemistry Departrment, Lucknow
University, Lucknow, U. P., India.
et. al carboxylates and the variety of metals for which such derivatives have been synthesized corresponding aryloxyace- tates are mainly confined to transition metal derivatives [11] with an occasional reference to organotin 12 and organo anti- mony [12, 13]derivatives. It has been found that etheral oxy- gen of aryloxyacetates group may or may not involve in co- ordination with metal atom and thus play a great role in shap- ing the geometry of the complex.
A perusal of the literature further reveals that the complexes of aryloxyacetic acids in case of organotin possess intermolecular O → Sn co-ordination, particularly for alkyltin derivatives and the compounds are monomeric in solid state. In sharp contrast to this triaryltin derivative having penta coordination around the tin were found to be polymer with bridging carboxylic group [12]. Although the aryloxy acetate of organophospho- rous and little known but the compound containing P-O, Sb- O, As-O have been studied to an appreciable extent [14, 15]. On the basis of ultraviolet and infrared spectra it has been concluded that aryloxyacetate behave as monodentate legend toward antimony (V) but slight decrease in IR frequencies for carbonyl group indelicate toward secondary interaction.
A comparison of spectral data of the organoantimony acetate with those of free legends both in solid state and in solution rules out the likelihood of co-ordination of antimony through etheral oxygen of the aryloxy group [9]. However, there seems to be no systematic and comparative study of aryloxyacetate derivative of antimony in +5 oxidation state as yet. In conclu- sion, in the solid state it indicates that sterically disfavored conformations of TBPY antimony (V) complexes may be stabi- lized by secondary bonding interaction involving diaxially co- ordinated ester legends. The complex geometry accompanied by the expansion of the co-ordination sphere from five to sev- en [23].
Continuing our interest in the carboxylate derivatives of group
15 elements [16, 20], a series of aryloxyacetate derivatives of
the general formula Ph3 Sb(OCOCH2 OR)2 and (p-MeO- C6 H4 )3 Sb(OCOCH2 OR)2 and Ph4 Sb(OCOCH2 OR) [where R = substituted aryl group; p-CH3 C6 H4 m-CH3 C 6 H4 – , O – CH3
C6 H4 – , b - C10 H7 -, O-Cl-C6 H4 ] has been synthesized and
characterized.
The main objectives of this work were aimed at:
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 29
ISSN 2229-5518
1. To synthesise and isolate hitherto unreported organo antimony (V) aryloxyacetate derivatives.
2. To investigate the role of etheral oxygen of aryloxy-
melting points after repeated crystallization as well as TLC run in chloroform – hexane mixture (1:1) with the observation of a single spot excluded the presence of mixture of reactants.
-3
acetate group, i.e., whether it participates in bonding
The molar conductance of 10
M solution were recorded in
or not and of carbonyl oxygen and thus raising the co-
methanol and were in the range of 15-25 ohm-1
, mole-1
cm2
ordination number of antimony (V) beyond five.
3. To ascertain the mode of bonding of aryloxyacetate
group toward antimony i.e., whether the acetate
group (s) act as monodentate or bidentate group or as a both.
The dual behavour of an acetate group as a monodentate or bidentate ligand has been well established in case of phenyl antimony (V) tetra acetate and dimethyl antimony (V) tri- acetate, where one acetate group is bidentate and the remain- ing act as a monodentate [15, 16]. Similarly, antimony in tet- ramethyl and –tetra phenyl antimony (V) acetate has been found to possess trigonal bypiramidal geometry in solution with the acetate group group acting as bidentate ligand. Thus it appears that it is the physical state and the nature and the number of organic groups attached to the antimony atom. Under anhydrous oxygen-free conditions, with absolute methanol as solvent, the reaction of tri (p-methoxy) antimony dichloride, triphenyl antimony dichloride or tetraphenyl an- timony chloride with silver salts of aryloxyacetic acids gave tri- and tetra-organo antimony (V) aryloxy acetates. The reac- tions with organoantimony (V) hailides with aryloxyacetic acids were also carried out in presence of triethyl amine as hydrogen halide acceptor in benzene. (Eq. 1-3).
Et3N
indicating the absence of ionic species in solution [21]. The
complexes were found to be monomeric in nitro benzene.
As expected infrared absorptions inherent to phenyl group (substituted i.e. p-tolyl) bound to antimony do not different appreciably from those values reported from parent (p- tolyl)3 Sb compounds, and hence not discussed here [22]. In- frared absorptions of diagnostic value for Phenyl antimony and p-tolyl antimony aryloxyacetates, related to the ligands have be identified which on preliminary stage indicate mode of bonding of aryloxy ligand. The data are presented in Table
3. The Characteristic v(OH) absorption bond of ligands which appeared around 3400 cm-1 in the free acid, was found mission in the newly synthesised complexes. A medium strong intensi- ty bond appearing at 1580-1690 cm-1 can confidently be as- signed to ν asy (OCO) mode whilst the comparatively weaker bond in the range 1360-1390 cm-1 can be attributed to ν sym (OCO) bond. The deformation mode as a medium intensity bond was found in the range 770 – 800 cm-1. The absorption associated with the antimony – oxygen appears in the range between 400 – 440 cm-1 and the absorption due to antimony carbon corresponding to y-mode occurs in the range 450-470 cm-1. These values clearly indicate the formation of phenyl; p- methoxyphenyl antimony (V) aryloxyacetates.
The comparison of IR spectra of the compounds with those of
(p-MeO-C6H4)3SbCl2 + 2ROCH2COOH
![]()
(p-MeO-C6H4)3SbCl2 + 2ROCH2COOAg
![]()
abs. alcohol
Benzene/THF
abs. alcohol
(p-MeO-C6H4)3Sb(OCOCH2OR)2 + 2Et3N.HCl (1)
(p-MeO-C6H4)3Sb(OCOCH2OR)2 + 2AgCl (2)
respective ligand in solid and solution shows a very slight
shift in ν asym (C = O), ν sym (C - O) and v(C - O - C) defor-
mation band which in turn showed the lack of co-ordination
antimony through - C = O center of the ligands but it repre-
Ph3SbCl2 + 2ROCH2COOH
Et3N
![]()
Ph3Sb(OCOCH2OR)2 + 3Et3N.HCl (3)
abs. alcohol
Benzene/THF
sents a slight presence of secondary interaction which indirect- ly provides strength to the sterically congested TBPY geome-
Ph3SbCl2 + 2ROCH2COOAg
![]()
Ph4SbCl + ROCH2COOH
abs. alcohol
Et3N
![]()
Ph3Sb(OCOCH2OR)2 + 2 AgCl (4)
try. Further it has been reported earlier that separation be-
tween [v(ν asy(OCO) – ν sym (OCO)] is smaller, around 150 cm-1
abs. alcohol Ph4Sb(OCOCH2OR) + Et3N.HCl (5)
in the case of linear polymeric moieties and is considerably
-1
[where R = p-CH3C6H4-, m-CH3C6H4-, C10H7-, o-ClC6H4, o-CH3C6H4-, Ph = C6H5-]]
larger (around 250 – 350 cm
) for monomeric compounds [16
– 20]. Since the separation observed in the present compounds
-1
All the reactions were performed under an atmosphere of dry
is fairly large (2300 cm
), monomolecular constitution seems
nitrogen. Tetrahydrofuran (THF) was distilled under an at-
mosphere of nitrogen from sodium benzophenone. Silver salts
of carboxylic acids were prepared from the corresponding
sodium salt by reaction with AgNO3 . The products were re-
crystallized from petroleum ether (400 - 600C) or benzene. The
complexes are off white to light brown solid and are generally
obtained as a sticky man which on treatment with sodium
dried benzene. Solidified and subsequently crystallized with
benzene/pet-ether. The complexes are fairly stable to air and
moisture and have sharp melting point. There is no regular
trend of the melting point of the complexes and they melt
without decomposition. Complexes are soluble in chloroform
and acetonitrile. They can be stored at room temperature
without decomposition for several weeks. The constancy in
to be most plausible where antimony would be having a co-
ordination number five. This observation is in sharp contrast
to organo tin complexes of aryloxyacetates which have been
found to be polymeric involving carboxylic bridges [12].
1H NMR spectra (Table 5) of the compounds were recorded in CDCl3 using TMS as an internal reference. The disappearance of -OH proton signal (δ 9.2 ppm) present in the ligand indicate the formation of aryloxy acetate derivative. The appearance of singlet for -CH2 - protons in compounds at δ 4.80-4.8: ppm showed that both the ligands are equivalent and seemed to be in one plane. The protons of phenyl group directly attached with antimony appears as multiplet in the range δ7.70 – 7.30 ppm, protons of o-tolyl group directly attached with antimony
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 30
ISSN 2229-5518
also appears as multiplet in the range δ7.35 – 7.00 ppm and of CH3 - group attached to oxygen atom of methoxy group ap- pears to shielded at δ 3.85 ppm. Protons of b-naphthyl group
anhydrous aluminium (III) chloride.
Neat/ AlCl3
![]()
Ar3Sb + ArX H. Temp.>200°C
Ar4SbX
also appears as multiplet in the range δ8.00 – 7.20 ppm and
-CH2 - group of acetate moiety appears at δ5.14 ppm. Protons
of ortho, Meta and para-cresol appears as multiplet in the
range δ 2.10 - 7.25 ppm and of - CH2 - appears at δ 5.10 ppm.
Protons of Ortho – Chlorophenoxy acetate appears as multi-
plet in the range δ 6.90 – 7.55 ppm and of - CH2 - at δ 5.12
ppm.
The 13C NMR (Table 6) of all the compounds were recorded in CDCl3 at 75 MHz on 300 MHz FT NMR (Bruker DRX – 300) instrument and the data are summarized in Table. In every case i – c δ(139.5 - 135.5) ppm of phenyl ring and i-c δ(129.5 -
135.5) ppm of p-MeO – phenyl ring (i.e. Sb – C) was found to be more deshielded than O - C; δ(135.5 - 137.5) ppm; m – C, δ (110.5 – 129.5) ppm; and p – c, δ (157.6 – 128.7) ppm. The me- thyl carbon of methoxy group of p-tolyl moiety directly at- tached to antimony appeared at δ(55.8 – 56.0) ppm. The b- naphthol aryloxy acetate moiety appears at δ(105.9 – 156.5) ppm. The ortho, meta, para cresol moiety of aryloxy acetate group appears in the range δ(110.5 – 160.5) ppm and CH3 – group appears at δ(15.5 – 22.5) ppm, in both cases the - CH2 - group of aryloxy acetate moiety appears in the range of δ(68.5
– 69.0) ppm.
Triphenyl antimony (V) dichloride Ph3 SbCl2 , tri (p-tolyl) an-
timony (V) dichloride (p-MeO-C6 H4 )3 SbCl2 were prepared by Grignard reaction followed by oxidative addition of chlorine and tetraphenyl stibonium (V) chloride was prepared by Quarternisation reaction as follow:
A solution of antimony trichloride (22.8g, 0.1mol) and 4-bromo anisole (47.1g, 0.1mol) in benzene (200 ml) was added drop wise to a boiling suspension of sodium (13.8g, 0.6mol) in the same solvent (300 ml). The reaction mixture was refluxed for
4h with occasional shaking and then filtered hot. The residue was extracted twice with hot benzene. The solvent was ex- tracted twice with hot benzene. The solvent was completely distilled and the remaining residue was recrystallised from alcohol-petroleum ether (60-80°C) mixture.
M.P.: 120°C Lit.: 120-121°C [25]
It was prepared by slowly passing freshly prepared chlorine for 30 minute through a solution of tris (p-tolyl) antimony (4.43g, 0.01mol) in carbon tetrachloride. Filtration and recrys- talization from the same solvent gave tris (p-tolyl) antimony (V) dichloride; (p-CH3 O-C 6 H4 ) 3 Sb.
M.P.: 156-157°C Lit.: 157°C [26]
Tetraaryl stibonium halide, Ar4 SbX, was conveniently pre-
A mixture of triphenyl antimony (7.0g, 0.02mol), anhydrous aluminium (III) chloride (7.0g, 0.05mol) and chlorobenzene (2.86g, 0.02mol) was taken in a dry round bottom flask with an air condenser. The reaction mixture was heated gradually on an oil bath up to 225-230°C in half an hour, and this tempera- ture was maintained for further 2h. The black oily mass was poured in one liter capacity beaker containing water (600ml) and boiled on wire gauge till complete black mass was disap- peared In to water leaving only little residue. Solution was filtered and the filtrate was concentrated to 1/4th of the original volume. Solution was cooled and then added KCl (5g,
0.04mol) to obtain white crystalline Tetraphenylstibonium
chloride, which was dried between pads and recrystallised from absolute alcohol.
M.P.: 215°C Lit.: 208-218°C [24]
Typical experimental details of the reactions are described be- low. Relevant IR and UV, NMR assignments and analytical data are summarized in Table 1-6.
To a stirring solution of tris (4-methoxyphenyl) antimony (v) dichloride (0.514 g; 1 mmol was added (2 – (O – tolyl – oxy) acetoxy) silver (0.546g: 2 mmol) in presence of benzene as sol- vent was stirred in anhydrous. Oxygen free conditions for 6h, followed by refluxing for 2h to ensure completion of the reac- tion. A Flocculent white ppt. of AgCl was formed which was filtered off. The filtrate on concentration gave a light brown solid which was recrystallized by petroleum ether (400 – 600 C).
6h. It was later refluxed for 2h to ensure completion of the re-
action. An off white precipitate of AgCl was formed which
was filtered off. The filtrate on concentration gave a light
brown solid which was crystallized from hexane and petrole-
um ether (400 – 600C) mixture.
mony (V) aryloxyacetates with possible weak secondary inter- action:![]()
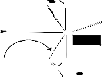
R'
C O Cl O
R - R
-2Cl
R Sb R Sb
R R
pared by Quarternisation reaction of triaryl stibine, Ar3 Sb, Cl
with aryl halide, ArX, in the presence of Lewis acid catalyst
O O
weak secondary C Interaction O
R'
Fig: Sterically disfavoured conformation of TBPY antimony(V) stablised by weak secondary bonding
Interaction involving diaxially co-ordinated aryloxy acetates
[Where R = C6H5- ; CH3O-C6H4- R' = b-naphthyl, o-cresol, m-cresol, p-cresol, o-Cl-phenyl]
aryloxyacetates
![]()
S.No. Complex Recrystalization solvent M.P.
(0C)
Colour
![]()
In1te(p-CHrOCnH ) Sb(aOCOCtH OiCoH (CHn)-O) al Jou0 r0 nal f Scientific & Engineering Research, Volume 4, Issue 7, July-2013 31
IS2S(pN-CH3OC6H4)23Sb(OC2OCH22OC6H49(CH3)--m)25H5exane1+ Pet.8ether(40 – 60 C) 128 Light Brown
3 (p-CH3OC6H4)3Sb(OCOCH2OC6H4(CH3)-p)2 Benzene 110 Light Brown
4 (p-CH3OC6H4)3Sb(OCOCH2OC10H7-b)2 Pet. Ether (400-600C) 125 Dirty White
5 (p-CH3OC6H4)3Sb(OCOCH2OC6H4(Cl)-O)2 Hexane – Benzene 140 White
6 (C6H5)3Sb(OCOCH2OC10H7-b)2 Benzene 135 Dirty White
7 (C6H5)3Sb(OCOCH2OC6H4Cl - O)2 Benzene 88 White
![]()
Table 4 IR Absorption frequencies of Organoantimony (V) arylox y acetates
58 T(C6H5)3SAb(OCOCH2BOC6H4(CH3)L- O)2 ESBenzene 148 White
S.No. Complex vasy(OCO) vsym
![]()
(OCO)
v(Sb – O)
v(Sb – C)
y mode
1 (p-CH3OC6H4)3Sb(OCOCH2OC6H4(CH3)-o)2 1680 1370 430 450
2 (p-CH3OC6H4)3Sb(OCOCH2OC6H4(CH3)-m)2 1685 1383 429 455
1675 1386 425 451
9 (C6H5)3Sb (OCOCH2OC6H4 (CH3) - p)2 Hexane 145 White
10 (C H ) Sb(OCOCH O C H (Cl) - O) Pet. Ether (400 – 600) 90 Light Brown
Tabl6 e5 4 – 12 :6 4 Preparation and Properties of Organoantimony (V)aryloxyacetates
11 (C6H5)4Sb(OCO CH2O C6H4(CH2) - O) Pet. Ether (400 - 600C) – Hexane 126 Light Brown
3 (p-CH3OC6H4)3Sb(OCOCH2OC6H4(CH3)-p)2
4 (p-CH3OC6H4)3Sb(OCOCH2OC10H7-b)2 1696 1380 425 456
5 (p-CH3OC6H4)3Sb(OCOCH2OC6H4(Cl)-o)2 1698 1389 430 470
6 (C6H5)3Sb(OCOCH2OC10H7-b)2 1685 1390 419 467
7 (C6H5)3Sb(OCOCH2OC6H4Cl - o)2 1703 1395 411 455
8 (C6H5)3Sb(OCOCH2OC6H4(CH3) - o)2 1675 1360 415 458
9 (C6H5)3Sb (OCOCH2OC6H4 (CH3) - p)2 1694 1360 417 451
10 (C6H5)4Sb(OCOCH2O C6H4(Cl) - o) 1650 1387 415 461
11 (C6H5)4Sb(OCO CH2O C6H4(CH2) - o) 1655 1375 411 465
![]()
12 (C6H5)4Sb (OCO CH2O C6H4(CH3) - p) 1694 1360 417 467
![]()
S12.N(Co6H5).4Sb (OCO CH2O C6H4(CH3) - p) Benzene
![]()
C200 omLightpBrowlnex Recrystalization solvent M.P. (0C)
Colour
![]()
0 0
1 (p-CH3OC6H4)3Sb(OCOCH2OC6H4(CH3)-O)2 Hexane + Pet. ether (40 – 60 C) 132 Light Brown
0 0
2 (p-CH3OC6H4)3Sb(OCOCH2OC6H4(CH3)-m)2 Hexane + Pet. ether(40 – 60 C) 128 Light Brown
3 (p-CH3OC6H4)3Sb(OCOCH2OC6H4(CH3)-p)2 Benzene 110 Light Brown
4 (p-CH3OC6H4)3Sb(OCOCH2OC10H7-b)2 Pet. Ether (400-600C) 125 Dirty White
5 (p-CH3OC6H4)3Sb(OCOCH2OC6H4(Cl)-O)2 Hexane – Benzene 140 White
6 (C6H5)3Sb(OCOCH2OC10H7-b)2 Benzene 135 Dirty White
7 (C6H5)3Sb(OCOCH2OC6H4Cl - O)2 Benzene 88 White
8 (C6H5)3Sb(OCOCH2OC6H4(CH3) - O)2 Benzene 148 White
9 (C6H5)3Sb (OCOCH2OC6H4 (CH3) - p)2 Hexane 145 White
0 0
10 (C6H5)4Sb(OCOCH2O C6H4(Cl) - O) Pet. Ether (40 – 60 ) 90 Light Brown
11 (C6H5)4Sb(OCO CH2O C6H4(CH2) - O) Pet. Ether (400 - 600C) – Hexane 126 Light Brown
![]()
12 (C6H5)4Sb (OCO CH2O C6H4(CH3) - p) Benzene 200 Light Brown
Table 5 1H NMR Spectra of Representative Organoantimony (V) Aryloxy Acetates In δppm
![]()
![]()
Comp.
No. CH3O- -O-C6H4 C6H5 -CH2’ - CH3 - -O-C6H4- -O-C10H7 -O
C2/C6 C3/C5 C2/C6 C3/C4/C5 C2/C6 C3/C5 -
Table 2 Elemental Analysis data
![]()
S.No. Complex Molar Conductance (in
Molecular Weigh
1 3.80-3.85 (S)
6.80-
7.45 (m)
6.90-
7.01 (m)
4.90-5.10
- - (s)
2.00-
3.00 (s)
6.80-
7.00 (m)
7.10- -
7.55 (m)
MeOH) (Ohm-1 mole-1
![]()
cm2)
Found (Calcd.)
4 3.75-3.90 (s)
6.75-
7.35
6.90-
7.00
4.90-5.15
- - (s)
- - - 7.20-7.99 (m)
1 (p-CH3OC6H4)3Sb(OCOCH2OC6H4(CH3) – o)2 15.01 772.51 (773.48)
2 (p-CH3OC6H4)3Sb(OCOCH2OC6H4(CH3) – m)2 16.5 771.51 (773.48)
3 (p-CH3OC6H4)3Sb (OCOCH2OC6H4(CH3) - p)2 17.5 772.51 (773.48)
4 (p-CH3OC6H4)3Sb (OCOCH2OC10H7 - b)2 21.02 844.30 (845.55)
5 3.80-3.89 (s)
(m)
6.70-
7.50 (m)
(m)
6.89-
7.12 (m)
4.19-5.25
- - (s)
- 6.70-
7.15 (m)
7.10-
7.85 (m)
- 7.1
5 (p-CH3O – C6H4)3Sb (OCOCH2O C6H4(Cl) -o)2 23.05 809.85 (814.32)
6 (C6H5)3Sb (OCOCH2OC10H7 - b)2 17.05 745.40 (755.47)
6 - - - 7.44-7.50 (m) 4.94.5.25
(s)
- - - 7.15-8.10
(m)
7 (C6H5)3Sb(OCOCH2OC6H4Cl - o)2 16.01 714.25 (724.24)
8 (C6H5)3Sb(OCOCH2O C6H4(CH3) - o)2 20.15 682.89 (683.40)
9 (C6H5)3Sb(OCOCH2OC6H4(CH3)3 - p)2 20.19 682.75 (683.40)
7 - - - 7.30-7.55 (m) 4.91-5.05
(s)
- 6.77-
7.17 (m)
6.10-
7.77 (m)
- 7.1
10 (C6H5)4Sb (OCOCH2OC6H4(Cl) - o) 17.61 612.78 (615.76)
11 (C6H5)4Sb (OCOCH2OC6H4(CH3) - o) 16.07 590.89 (595.34)
![]()
12 (C6H5)4Sb (OCOCH2OC6H4(CH3) - p) 20.14 594.38 (595.34)
8 - - - 7.45-7.55 (m) 4.90-5.85
(s)
11 - - - 7.35-7.55 (m) 4.85-5.25 (s)
![]()
Where, S = singlet, d = doublet, t = triplet, q = quatret and m = multiplet
2.10-
2.55 (s)
2.10-
2.75 (s)
6.00-
7.10 (m)
6.85-
7.10 (m)
6.00- -
7.15 (m)
6.15- -
7.01 (m)
![]()
Table – 6 13C NMR Spectra of Representative Organoantimony (V) Aryloxy Acetates
Comp. No.
R-Sb Ligands
![]()
CH3O- -O-C6H4 C6H5 -CH3 CH3
-O-C6H4- -O-C10H7
- -
i-C C2/C6 C3/C5 i-C C2/C6 C3/C4/C5 C2/C6 i-C C3/C5
1 54.8 130.5 136.5 112.5 - - - 65.5 15.8 112.5 155.5 130.5 -
![]()
Table 3 Elemental Analysis data of Organoantimony (v) Aryloxyacetates
4 55.6 132.5 136.8 118.5 - - - 67.5 - - - - 155.8
S.No. Complex Empirical
Formula
Found (Calcd.)%
![]()
C H
5 54.9 133.5 138.5 115.8 - - - 60.8 - 110.5 156.5 139.5 -
6 - - - - 135.5 136.5 128.5 70.1 - - - - 158.9
1 (p-CH3OC6H4)3Sb(OCOCH2OC6H4(CH3)-o)2 C39H39O9Sb 60.51(60.56) 4.05 (5
2 (p-CH3OC6H4)3Sb(OCOCH2OC6H4(CH3)-m)2 C39H39O9Sb 60.01 (60.56) 4.15 (5
3 (p-CH3OC6H4)3Sb(OCOCH2OC6H4(CH3)-p)2 C39H39O9Sb 59.80 (60.56) 4.98 (5
4 (p-CH3OC6H4)3Sb(OCOCH2OC10H7-b)2 C45H39O9Sb 62.87 (63.92) 3.95 (4
5 (p-CH3OC6H4)3Sb(OCOCH2OC6H4(Cl)-o)2 C37H33Cl2O9Sb 53.12 (54.57) 4.00 (4
6 (C6H5)3Sb(OCOCH2OC10H7-b)2 C42H33O6Sb 66.11 (66.77) 4.15 (4
7 (C6H5)3Sb(OCOCH2OC6H4Cl - o)2 C34H27Cl2O2Sb 55.19 (56.39) 3.61 (3
8 (C6H5)3Sb(OCOCH2OC6H4(CH3) - o)2 C36H33O6Sb 61.89 (63.27) 3.98 (4
9 (C6H5)3Sb (OCOCH2OC6H4 (CH3) - p)2 C36H33O6Sb 61.89 (63.27) 3.98 (4
10 (C6H5)4Sb(OCOCH2O C6H4(Cl) - o) C32H26ClO4Sb 60.85 (62.42) 4.10 (4
11 (C6H5)4Sb(OCO CH2O C6H4(CH2) - o) C33H29O3Sb 65.51 (66.58) 3.98 (4
![]()
12 (C6H5)4Sb (OCO CH2O C6H4(CH3) - p) C33H29O3Sb 65.01 (66.58) 4.01 (4
7 - - - - 139.5 140.5 130.5 69.5 - 114.5 158.5 126.8 -
8 - - - - 140.5 132.5 125.9 68.5 18.5 120.5 160.5 126.9 -
![]()
11 - - - - 138.5 135.5 130.1 68.7 16.10 118.5 162.5 135.5 -
where o=ortho, O= oxygen atom
![]()
![]()
5 6
H3CO 4
![]()
![]()
5 6
i Sb(OCOCH2'-R)2 4 i
3
![]()
![]()
5 6
Sb(OCOCH2'-R)2 ; i
3 4
Sb(OCOCH2'-R)
4
i = ipso carbon
![]()
3 2
O
i Cl
6 2
![]()
3 2 3 2
O O C
![]() 1 8
1 8
![]()
O i 9 7 O
-O-C6H4-Cl-o =
-O-C10H7 = CH3- of o/m/p cresol = CH3
5 3
4
IJSER © 2013
3 10 6
4 5
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 32
ISSN 2229-5518
Based on IR, UV and NMR spectral data enumerated above, it may tentatively be concluded that aryl oxyacetate under the present study behave as monodentate ligand having character of weak secondary interaction towards antimony in +5 oxida- tion state. Conductance measurement and molecular weight data showed that these aryloxyacetates have monomeric con- stitution and are nonconducting.
The authors are thankful to Head, Department of Chemistry, University of Lucknow, Lucknow for providing necessary la- boratory facility and University Grant Commission, New Del- hi, India for providing financial assistance through a Major Research Project vide letter No. 37-429/2009/ SR.
[23] Hubert Baruki, Simon J. coles, J.F. Costello, T. Gelbrich and M.B.
Hursthouse, DALTON, (2000).
[24] J. Chatt and F. G. Mann, J. Chem. Soc., 1, 192, (1940). [25] S. P. Olifirenko, Chem. Abstr., 63, 8401, (1965).
[26] J. Chatt and F. G. Mann, J. Chem., Soc., 1182, (1940).
[27] K. Singhal, R. Rastogi, P. Raj, Indial Journal of Chemistry, v- 26A, pp. 146-150, 1987.
[28] K. Singhal, P. Raj, Synth. React. Inorg. Met-Org. Chem., 23(6), pp.
1011-1020, 1993.
[29] F. Jee, A. K. Aggarwal, P. Raj, Proc. Indian natn. Sci. Acad, 59, A, 3, pp 309-313, 1993.
[30] G. B. Deacon, W. Roy, J. and J. M. Pfieffer, Aust. J. Chem., 37, pp
527-535, 1984.
[31] T. Hasan, P. K. Singh, R. Mishra, P. Raj, N. Misra, Pramana Journal of Physics, v-68, 5, pp. 875-880, 2007.
[32] Th. Klapotke, Journal of Organometallic Chemistry, 331, pp. 299-
307, 1987.
[33] S. Agnihotri, P. Raj, K. Singhal, Synth. React. Inorg. Met-Org.
Chem., 32 (3), pp. 449-464, 2002.
[34] P. Raj, S. Agnihotri, K. Singhal, Synth. React. Inorg. Met-Org.
Chem., 32 (3), pp. 569-581, 2002.
[1] R.C. Mehrotra and R. Bohra, Metal Carboxylates, Academic Press, London, 1983 and references there in.
[2] S.P. Bone and D.B. Sowerby, J. Organo met. Chem., 184, 181, 1980.
[3] M. Wieber, I. Fetzer-kremling, H. Reith and Burschka, Z. Natuforrch.
Teil B, 42, 815, 1987.
[4] R.G. Goel, Can. J. Chem., 47, 4607, 1969.
[5] V.H. Schmidbouer, R.H. Mitschke and S. Weidlein, Z.. Anorg. Allg.
Chem., 386, 146, 1971.
[6] M. Hall, D.B. Sowerby and C.P. Falshaw, J. Organo met. Chem. 315,
321. 1986.
[7] H.J. Frohn and H. Maurer, J. Fluorine Chem., 343, 129, 1986.
[8] G.S. Harris, A. Khan and I. Lennon, J. Fluorine Chem., 37, 247, 1987. [9] A.K. Saxena, A. Ranjan and P.S. Venkataramani, J. Fluorine Chem.,
64, 107, 1993.
Table – 1: Preparation and Properties of Organoantimony (V)
aryloxyacetates
![]()
S.No. Complex Recrystalization solvent M.P. (0C)
Colour
[10] H.A. Meinema and J.G. Noltes, J. organome chem., 313, 1972.
![]()
1 (p-CH OC H ) Sb(OCOCH OC H (CH )-O) 0 0
3 6 4 3
2 6 4 3
2 Hexane + Pet. ether (40 – 60 C) 132 Light Brown
[11] G. Smith, E.J. O' Reilly, C.H.L. Kennard, K. stadnaka & B
2 (p-CH3OC6H4)3Sb(OCOCH2OC6H4(CH3)-m)2 Hexane + Pet. ether(40 – 60 C) 128 Light Brown
0 0
Oloeksyn, Inorg. Chim. Acta, 47, 11, 1981.
3 (p-CH3OC6H4)3Sb(OCOCH2OC6H4(CH3)-p)2 Benzene 110 Light Brown
0 0
[12] T.K. Chattopadhyay, A.K. Kumar, B. Majee, Ind. J. Chem., 33A ,
948, 1994.
[13] N. Sharma, B.S. Golen, R.K. Mahajan, S.C. Chaudhri, Polyhedron,
10, 789, 1991; V.K. Jain, R. Bohra and R.C. Mehrotra, Struct. Bond,
4 (p-CH3OC6H4)3Sb(OCOCH2OC10H7-b)2 Pet. Ether (40 -60 C) 125 Dirty White
5 (p-CH3OC6H4)3Sb(OCOCH2OC6H4(Cl)-O)2 Hexane – Benzene 140 White
6 (C6H5)3Sb(OCOCH2OC10H7-b)2 Benzene 135 Dirty White
7 (C6H5)3Sb(OCOCH2OC6H4Cl - O)2 Benzene 88 White
8 (C H ) Sb(OCOCH OC H (CH ) - O)
6 5 3
2 6 4 3
2 Benzene 148 White
52, 147, 1982.
9 (C H ) Sb (OCOCH OC H (CH ) - p)
Hexane 145 White
6 5 3
2 6 4 3 2
[14] Ashok Ranjan, A.K. Saxena, P.S. Venkataramani, Ind. J. Chem., 33A,
10 (C6H5)4Sb(OCOCH2O C6H4(Cl) - O) Pet. Ether (40 – 60 ) 90 Light Brown
0 0
948, 1994.
[15] Ajita Pandey, "Studies on Organoarsenic (v) and organo-antimony
(v) derivatives", Ph.D. Thesis, Lucknow University, Lucknow,
1998; R.R. Holmes "Penta co-ordinated Phosphorous structure and spectroscopy" vol.1, ACS manager, Washington D.C. 1980.
[16] Prem Raj, A.K. Aggarwal, Kiran Singhal, Synth. React. Inorg. Met.
Org. Chem, 22(5), 509, 1992.
[17] Prem Raj, A.K. Saxena, Kiran Singhal, A.Ranjan, Polyhedron, 4(2),
251, 1985.
[18] Kiran Singhal, Rajiv Rastogi, P.Raj, Ind. J. Chem., 26A, 146, 1987.
[19] Prem Raj, Ashok Ranjan, Kiran Singhal, Rajiv Rastogi, Synth.
Synth. React. Inorg. Met. Org. Chem, 14(2), 269, 1984.
[20] Prem Raj, K. Singhal, S. Agnihotri, Synth React Inorg, met. org. chem.
2001.
[21] W.J. Geary, Coord. Chem. Rev., 7, 81, 1971.
[22] E. Maslowsky (Jr.) Organomet. chem., 70, 153, 1974.
11 (C6H5)4Sb(OCO CH2O C6H4(CH2) - O) Pet. Ether (400 - 600C) – Hexane 126 Light Brown
![]()
12 (C6H5)4Sb (OCO CH2O C6H4(CH3) - p) Benzene 200 Light Brown
IJSER © 2013 http://www.ijser.org