International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1132
ISSN 2229-5518
Suspended solid growth method for the re-
moval of organic matter from industrial ef- fluent
Pratibha R.Gawande1,Kalyan Patil2,Dr.Kalpana S.Deshmukh
Abstract- The purpose of this this research works, in order to study the effectiveness of activated sludge process at laboratory scale basis. Activated sludge process is the biological aerobic wastewater treatment that uses micro-organisms and air to biolog- ically oxidize the organic pollutants. This uses naturally occurring bacteria and protozoa thus it is eco-friendly as well as eco- nomical process. Activated sludge process was found to be effective method with scope for further research in terms of cost ef- fectiveness, good quality effluent, efficient removal of BOD and COD.
A study was conducted to evaluate the feasibility of Activated Sludge Process (ASP) for the treatment of tannery wastewater and to develop a simple design criteria under local conditions. A bench scale model comprising of an aeration tank and final clarifier was used for this purpose. The model was operated continuously for 30 days. Settled sugar industry and textile indus- try wastewater was used as influent to the aeration tank. Five days Biochemical Oxygen Demand (BOD5) and Chemical Oxygen Demand (COD) of the influent and effluent were measured to find process efficiency at various mixed liquor volatile suspended solids (MLVSS) and hydraulic detention time (θ). The results of the study demonstrated that an efficiency of above 90% and 80% for BOD5 and COD, respectively could be obtained if the ASP is operated at an MLVSS concentration of 3500 mg/L keeping an aeration time of 10 hours.
Index Terms— Activated sludge process, BOD5,COD. laboratory scale process, organic matter.
1 INTRODUCTION
—————————— ——————————
Advancement in needs and technology has necessitated the evolving of treatment processes that remove dissolved matter and toxic substances. Currently, the advancement of scientific knowledge and moral awareness has led to a reduction of dis- charges through pollution prevention and recycling, with the noble goal of zero discharge of pollutants. Treatment technol- ogy includes physical, biological, and chemical methods. Re- sidual substances removed or created by treatment processes must be deal with and reused or disposed of in a safe way. The purified water is discharged to surface water or ground water. Residuals, called sludge or bio solids, may be reused by carefully controlled composting or land application. Some- times they are incinerated.
We cannot allow wastewater to be disposed of in a manner
dangerous to human health and lesser life forms or damaging
to the natural environment. Our planet has the remarkable
ability to heal itself, but there is a limit to what it can do, and
we must make it our goal to always stay within safe bounds.
That limit is not always clear to scientists, and we must al-
ways take the safe approach to avoid it. Basic wastewater
treatment facilities reduce organic and suspended solids to
limit pollution to the environment.
————————————————
• Pratibha R.Gawande is currently pursuing masters degree program in Chemi- cal engineering in shivaji university ,Kolhapur,India, E-mail: pratibharga- wande@rediffmail.com
• Kalyan patil working as a associate professor in Tatyasaheb kore institute of
engineering and technology,Kolhapur,India
• Dr.Kalpana S.Deshmukh is working as a professor in Datta meghe college of
engg,Airoli,Navi Mumbai,India
Activated sludge refers to a mass of microorganisms culti-
vated in the treatment process to break down organic matter
into carbon dioxide, water, and other inorganic compounds.
The activated sludge process has three basic components: 1) a reactor in which the microorganisms are kept in suspen- sion, aerated, and in contact with the waste they are treating;
2) liquid-solid separation; and 3) a sludge recycling system for returning activated sludge back to the beginning of the pro- cess. There are many variants of activated sludge processes, including variations in the aeration method and the way the sludge is returned to the process.
While many activated sludge treatment works have been built in developing countries, very few work as well as in- tended. Activated sludge can be appropriate where high re- moval of organic pollution is required, funds and skilled per- sonnel are available for operation and maintenance, and land is scarce or expensive. Since activated sludge requires the con- tinuous operation of oxygen blowers and sludge pumps, a steady energy supply is a key requirement. The system usual- ly needs some form of pre-treatment, such as screening and primary sedimentation.
Efficient removal of BOD, COD and nutrients when de- signed and professionally operated according to local re- quirements. The process itself has flexibility and numerous
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1133
ISSN 2229-5518
modifications can be tailored to meet specific requirements (e.g. for nitrogen removal). Activated sludge is the best doc- umented and most widely used form of secondary wastewater treatment. Among the raw material sources for distillery, two very important raw materials are cane sugar molasses and beet sugar molasses. Distillery waste water (stil- lage) is the main by product originating in the distilleries, and its volume is 10 times of ethanol produced.. It is not surpris- ing that the utilization of the stillage raises serious problems, and that many attempts have been made all over the world to solve them.
Distillery wastewater is usually comprised of high volume of greatly acidic matter which presents many disposal and treatment problems. Waste streams generally contain high levels of both dissolved organic and inorganic materials. The treatment of the effluent for removal of organic matter can be carried out by various chemical and biological methods. Still there is potential for adoption as an important method for it.
There is a vast literature on the design of various forms of the activated sludge treatment process. General considera- tions include: wastewater characteristics, local environmental conditions (including temperature), possible presence of toxic or other inhibitory substances (will the process receive indus- trial effluents or septage, for instance), oxygen transfer re- quirements and reaction kinetics (detention time in the sys- tem, related to quality and quantity of wastewater received, effluent requirements, sludge treatment requirements and other factors listed
2 II.SUSPENDED SOLID GROWTH METHOD
A].Literature review
Adaptation of bagasse flyash, a sugar industry solid waste into zeolitic material for the uptake of phenol was studied by Shah et al. [1]. Ahamaruzzaman [2] has studied Role of flyash in removal of organic pollutants from wastewater. Removal of dissolved organic matter by granular-activated carbon adsorp- tion as a pretreatment to reverse osmosis of membrane biore- actor. Bioreactor effluents was studied by Reznik et. al. [3]. Marin and. Beiras [4] have carried out work on adsorption of different types of dissolved organic matter to marine phyto- plankton,Matilainen et al.[5)presented coagulation and floccu- lation as effective methods for removal of natural harmful or- ganic matter from water. Ren et. al. [6]experimentally reported the application of carbon nanotubes (CNTs) as a new type of adsorbents for the removal of various inorganic and organic pollutants, and radionuclides from large volumes of wastewater. Hami et al.[7] investigated effective use of pow- dered activated carbon (PAC) on the performance of a pilot- scale laboratory dissolved air flotation (DAF) unit. Experi- mental findings shows that for dosages of activated carbon in the range of 50–150 mg/l, the removal efficiencies for BOD increased from 27–70% to 76–94% while those for COD in- creased from 16–64% to 72–92.5% for inlet values of 45–95 mg/l and 110–200 mg/l for BOD and COD respectively. Al- varez et al[8]. reported the application of a simultaneous com- bination of ozone and granular activated carbon as a tertiary treatment of a wastewater generated from the activity of vari-
ous food-processing industries.
Chaudhari et al.[9] carried out experimental investigations on the removal of molasses-derived color and chemical oxy- gen demand from the biodigester effluent of a molasses-based alcohol distillery effluent treatment plant using inorganic co- agulants. Flocculation with carbon was found to be a better alternative to the conventional aerobic treatment process of the biodigester effluent. Mohana et al[10. presented an over- view of the pollution problems caused by distillery spent wash, the technologies employed globally for its treatment and its alternative use in various biotechnological sectors. Satyawali and Balakrishnan [11]presented a review of the existing status and advances in biological and physico- chemical methods.Hyung and Kim [12] have used natural or- ganic matter for adsorption to multi-walled carbon nanotubes and also observed the effect of natural organic matter charac- teristics and water quality parameters. COD is the amount of oxygen consumed for oxidizing the organic matter. In the pre- sent study the oxidizing agent used is potassium dichromate. Oxygen consumption values can be used to quantify the amount of organic matter present in the wastewater. The typi- cal COD and BOD of the distillery effluent are 70000-100000 and 45000-60000 respectively. By using primary and second- ary treatment it can be brought down to 5000 – 8000. For fur- ther removal of organic matter many advanced methods have been tried. The UASB reactor for post treatment of distillery waste water was reported by Musee et. Al[13].A Distillery spentwash was treated in the hybrid anaerobic reactor by Gupta and Singh.[14] Natraj et, al [15]2006,Chemical oxygen demand was considerably reduced in distillery wastewater in India in order to reduce the cost of waste water disposal. This process emphasized the recovery and recycling of valuable chemicals contained in waste water.Coetzee et.al[16]the aero- bic treatment systems are used mainly to remove the BOD of the waste .The partial reduction of BOD is achieved in many distilleries using biological treatment.Wentzel et,al[17].(1985)developed effluents generated from the distill- ery were treated in two stage anaerobic-aerobic biological sys- tem. This set up has been developed for purpose of treating winery effluent.Borja et.al.[18]1993 Seghezzo Etal 1998,in addi- tion to COD and BOD pollution wine distillery wastewater contain phenolic compounds mainly gallic acid,pcoumaric acid and gentisic acid which is impart high antibacterial activi- ty.[19]
B] Objective of work
The main objective of this text is to offer the reader the neces- sary tools for the design and optimization of activated sludge processes for both municipal- and industrial waste waters. Now- adays, these processes will in general include tertiary treatment and anaerobic sludge digestion. A simplified quantitative steady state model is presented that will prove extremely useful in the design and optimization of activated sludge systems and auxilia- ry units such as final settlers, thickeners and sludge digesters
The model describes the removal of organic material in the activated sludge system and its consequences for the principal parameters of the process: effluent quality, excess sludge produc- tion and oxygen consumption. It has been extended to include
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1134
ISSN 2229-5518
both nitrogen- and phosphorus removal. The text will also deal with operational problems of activated sludge systems: e.g. sludge settling and -thickening, oxygen transfer, maintenance of an adequate pH, sludge digestion.
The aim of the present work is to use suspended biological processes i.e. Activated sludge process for removal of organic matter from distillery effluent and also optimizing various pa- rameters. These Studies will focus on:
• Role of activated sludge and nature of the removal process.
• Studies will be carried out with or without aeration of the acti- vated sludge to study the effect of oxidation.
• To study optimization of parameters such as solid concentra- tion of effluent, inlet concentration of effluent and pH.
• To study various values of these parameter and optimum value of parameter.
• Know the major components of an activated sludge wastewater treatment system
• Be able to calculate required aeration tank volume (in S.I. units) for a specified volumetric loading, hydraulic residence time, or aeration tank F:M ratio, if given suitable aeration tank influent and aeration tank parameter information
• Be able to calculate the required activated sludge recycle flow rate, waste activated sludge flow rate, and aeration tank F:M ratio, if given suitable wastewater stream and aeration tank in- formation along with the desired value for sludge retention time
III.EXPERIMENTAL WORK (MATERIAL AND METHODS)
I)Effluent collection and analysis
• Effluent was from Sahakari Sakhar Karkhana Pvt.Ltd, Pune which produces sugar as its main product from sugarcane and used in Activated Sludge process..
• The effluents (SME) were collected from outlet of the second- ary settling tank installed in the campus of Sugar mill to re- duce the BOD and solids using plastic container. The sugar mill effluent was brought to the laboratory and was analyzed for various physico-chemical and microbiological parameters viz. TS, TDS, TSS, EC, turbidity and pH, DO, BOD, COD, TKN, P, K+, Ca2+, Mg2+, Cl-, and HCO3– CO32-, Fe, Zn, Cd, Cu, Cr and Pb content following standard method.
• Characteristics of Effluents
• The characteristics of individual and combined effluents vary from mill to mill and from time to time. All the individual ef- fluents excluding spray pond overflow are acidic and coloured posters disagreeable older, high BOD and suspended solid. The oil and grease content is also high.
• The characteristics of effluents of a typical sugar mill are given in the following table.
• The activated sludge was taken from the wastewater treatment plant the sludge samples were brought immediately to the la- boratory, located at after sampling and the experiments were carried out directly thereafter
• The analyzed parameters for the study
• Physical Parameters :pH, EC, TDS, Temperature
• Physico-chemical Parameters :DO, COD, BOD
IV.Experimental Setup
Activated sludge – sludge particles produced in wastewater by the growth of organisms in aeration tanks. The term ‘activated’ comes from the fact that the particles teem with bacteria, fungi, and protozoa. Activated sludge is different from primary sludge in that the sludge particles contain many living organisms that can feed on the incoming wastewater. Activated sludge process is
a biological wastewater treatment process which speeds up waste decomposition. Activated sludge will be added to wastewater, and the mixture will be aerated and agitated. After a certain amount of time, the activated sludge will be allowed to settle out by sedimentation and will be disposed of (wasted) or reused (re- turned to the aeration tank).
An activated sludge process will consists of
• An aeration tank where the biological reactions occur.
• An aeration source that provides oxygen and mixing.
• A tank, known as the clarifier, where the solids settle and are separated from treated waste water.
• A means of collecting the solids either to return them to the aeration tank, (return activated sludge [RAS], or to remove them from the process (waste activated sludge [WAS].
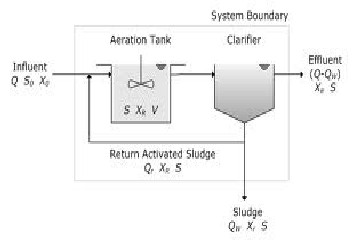
Following Parameters will be optimized during the study
• Removal efficiency of COD, BOD with activated sludge with aeration and without aeration at different contact time: The contact times provided will be 6, 12,18,24,30 hours with aera- tion.
• Effect of initial COD, BOD concentration on removal effi- ciency with aeration and without aeration: Samples with different initial concentrations will be used for experiment with different initial COD concentrations.
• Effect of pH on COD, BOD removal with aeration and without aeration: The experiments will be carried out at dif- ferent initial pH values. The pH values will be 2,4,6,8 and 10 with optimum contact time.
• Effect of biomass loading on COD, BOD removal with aer- ation and without aeration: The experiments for COD re- moval will be carried out at different biomass loading. The bi- omass loading taken will be 0.25, 0.5,1,2,3,4,5 grams/l.
VI.Wastewater Analysis
Various parameters considered while treating effluent are as fol- lows:
1). pH :
Tested using pH paper.
2).Biological Oxygen Demand: Principle:
Sufficient quantity of distilled water is aerated with aerator air compres-
sor for more than 8-10 hours and the aerated water is kept at low temper-
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1135
ISSN 2229-5518
ature till use. At the time of experiment this water is used for preparation of different dilution of the sample.
Some common ranges of BOD results are as follows, in mg/L:
Influent 150-400
Primary Effluent 60-160
Secondary Effluent 10-60
Digester Supernatant 1000-4000+ Industrial Wastes 100-3000+
Procedure
Preparation of solution:
Phosphate buffer: Dissolve 8.5 g KH 2 P04 , 21.75 g K 2HP04 , 33.4 g
Na2 HP04 •7H 2 0, and
1.7 g NH 4 Cl in approx. 500 ml reagent water. Dilute to 1 L. The pH should be 7.2. Store in 4°C refrigerator. Check before each
use for contamination (if there is any indication of biologi- cal/microbial growth, discard remaining reagent and prepare fresh).
• This will give blue colour. Continue titration to the disappear- ance of the blue colour. Record the final burette reading B1. Repeat the same procedure for Blank.
• Determine the DO of blank and sample after incubation for 5
day.(fifth day).Repeat the procedure.
• Note down initial and final reading.
• Take BOD bottle containing sample. Repeat the procedure for blank. Note down initial and final reading.
•
•
Calculation
(D0 -D5-BC) × Volume of diluted sample
BOD = Volume of sample taken
Where,
D0 : is the initial DO of diluted sample
D5: is the DO at the end of 5 days
BC: is the blank correction
Magnesium sulfate solution: Dissolve 22.5 g MgSO4•7H 2 0 in reagent water. Dilute to 1 L.
C0;
is the initial DO of the blank sample
Calcium chloride solution: Dissolve 27.5 g CaCl2 in reagent wa- ter. Dilute to 1 L.
Ferric Chloride solution: Dissolve 0.25 g FeCl3 •6H 2 0 in reagent water. Dilute to 1 L.
Sodium sulphate solution0.025N: Dissolve 1.575g Na2SO3 in distilled water and dilute to 1 liter. Solution should be prepared daily.
Determination of COD of sample:
• Prepare dilute mixture by adding sample in dilution water.(0.1-
1 for strong treated waste).
• Fill up one 300 ml bottle with the mixture and one with dilu- tion water (blank) in two set
• .Keep one set in BOD incubator for 5 days for incubation at 20
oc.
• Determine the DOof blank and sample immediately before in- cubation (first day).
• Determine the DO of blank and sample after incubation for 5 day.(fifth day).
• Repeat the procedure.
• Note down initial and final reading.
• Take BOD bottle containing sample. Repeat the procedure.
• Note down initial and final reading.
• Take BOD bottle & collect 300 ml of water sample into it.
• Add 2 ml of manganese sulphate by pipette & 2 ml of alkali potassium iodide solution to the BOD bottle.
• Insert stopper and shake bottle vigoursly.The top of the pipette should below the Liquid level, while adding these agents.
• Red precipitate will form if DO is present in water.
• Allow the precipitate to settle half way and mix again. Again allow the precipitate to settle half away.
• Add 2ml of concentrated H2SO 4. Allow the solution to stand at least 5 min to ensure formation of I2 which is to be titrated against sodium thiosulphate.
• A volume of 20ml is taken to conical flask & titrated with
0.05N sodium thiosulphate solution until yellow colour iodine turns to pale straw colour(very light).Since it is impossible to accurately titrate the sample to colourless liquid ,Add I ml of starch solution .
C5: is the DO at the end of 5 of the blank sample
3).Chemical Oxygen Demand
Principle:
Most of the organic matters are destroyed when boiled with a mixture of potassium dichromate and sulphuric acid producing carbon dioxide and water. A sample is refluxed with a known amount of potassium dichromate in sulphuric acid medium and the excess of dichromate is titrate against ferrous ammonium sul- phate. The amount of dichromate consumed is proportional to the oxygen required to oxidize the oxidizable organic matter.
The COD is usually defined as amount of O2 used while oxi- dizing matter of sample with strong chemical oxidants under
acidic conditions. Since, in the COD determination the organic (both biologically oxidisable like glucose and biological inert like cellulose) is completely oxidized to CO2 and H 2 O. The COD val- ues are greater than BOD values (which represent amount of oxy- gen and bacteria needed for stabilizing biologically oxidisable matter. The main advantage of the contact is that the determina- tion is completed in three hours compared to the 5 days required for the BOD determination and therefore steps can be taken to correct the errors on the day they occur.
The suitable amount of sample is boiled in known excess of K 2 Cr2 O7 in the presence of concentrated H2 SO4 . The organic matter is oxidized to CO2 and H 2 O.The excess of dichromate left unused is titrated with ferrous ammonium sulphate (FAS) stand- ard solution using ferroin indicator. The amount of K 2 Cr2 O7 con- sumed corresponds to COD. The dichromate reflux is preferred for the procedure using other oxidants because of superior oxidiz- ing ability, applicability to a wide variety of samples and ease of manipulation.
Procedure
Sample with COD of more than 50 mg O2/lit.
Preparation of dichromate solution (0.25 N): 12.25 gm of K2Cr2O7 is added to distilled water and volume is made upto 1 litre. Normality of solution is 0.1N
Preparation of Mohr’s salt solution :( 0.1N):39.2 gm of Fe (NH4)2(SO4)2.6 H2O is added to distilled water, 20 ml of concen- tric H2SO4 (3.6N) is added and volume is made 1000 ml with dis- tilled water to make 0.1 N Mohr’s solution.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1136
ISSN 2229-5518
Standardization of Mohr’s salt solution: 5 ml of K2Cr2O7 solu- tion (0.25N) is added to 45 ml of distilled water and 15 ml of conc.H2SO4 is added and this solution is cooled under tap water and titrated with Mohr’s salt solution in burette. Use ferroin indi- cator. Hence actual strength of Mohr’s salt is determined. Collection of waste H2O sample: Take sample in the flask. Determination of COD of sample:
• Sample water is collected and the following setup is round bot-
tom flask with glass joints. To the water sample following rea- gents are added in sequential order. (a). 0.5 gm of HgSO4(Mercuric sulphate)(b)10 ml of K2Cr2O7(0.25N)(c)20 ml conc. H2SO4 (d) 0.5 gm Ag2SO4(silver sulphate)(e) 10 ml of water sample. In case sample water is very dirty, we should add less volume of sample water diluted with 10 ml distilled water.
• The blank set is arranged with same reagent added in the same order except that 10 ml of distilled water replaces 10 ml of sample water.
• The blank set and the sample are refluxed for 3 hrs. The sides of condenser are then washed with approximately10 ml of wa- ter and cooled.
• The excess of K2Cr2O7 is titrated with std. Mohr’s salt solu- tion using few drops of ferroin as indicator.
• Near the end point of the titration color changes sharply from green blue to wine red. Reflux blank simultaneously with the sample under identical conditions.
• Place 0.4g HgSO4 in a reflux tube.
• Add 20ml or an aliquot sample diluted to 20 ml with distilled
water. Mix well, so that chlorides are converted into poorly ionized mercuric chloride.
• Add 10ml standard K2Cr2O7 solution and then add slowly 30 ml sulphuric acid which already containing silver sulphate.
• Mix well, if the colour turns green, take a fresh sample with smaller aliquot.
• Final concentration of concentrated H2SO4 should always
18N. Connect the tubes to condenser and reflux for 2 h at
150oC.
• Cool and wash down the condensers with 60ml distilled water.
• Cool and titrate against standard ferrous ammonium sulphate using ferroin as indicator.
• Near the end point of the titration color changes sharply from green blue to wine red.
• Reflux blank simultaneously with the sample under identical conditions.
Calculation
Procedure:
• Take a known volume of a well-mixed sample in a tarred dish ignited to constant weight (W1) & take weight of waste water with porcelain dish (W2).
• Evaporate the samples to dryness at 103-1050C for 24 hrs.
• Cool in desiccator weigh and record the reading (W3).
• Ignite the dish partially in air until most of heat has been dissi-
pated and then transfer to a desiccator for final cooling in a dry atmosphere and record final weigh (W4).
Total dissolved solids (mg/L) Total dissolved solids (mg/L)
= (W4-W3)*1000*1000
—―――――———— Volume of sample in ml
Where, W3=Weight of empty porcelain dish
W4= Weight of empty porcelain dish with sample
= (W4-W3)*1000*1000
—―――――———— Volume of sample in ml
Where, W3=Weight of empty porcelain dish
W4= Weight of empty porcelain dish with sample
5) Total Suspended Solids
Principle:-
A Mixed Liquor Suspended Solids (MLSS) is a test for the total suspended solids in a sample of mixed liquor. This test is essen- tially the same as the test you performed for TSS in the last lab, except for the use of mixed liquor as the water sample. In addi- tion, the concentration of suspended solids found in the mixed liquor is typically much greater than that found in the raw or treated water. MLSS concentrations are often greater than 1,000 mg/L, but should not exceed 4,000mg/L. Well-mixed sample is filtered through a weighed standard glass-fiber filter and the residue retained on the filter is dried to a constant weight at 103 to 105°C. The increase in weight of the fil- ter represents the total suspended solids. If the suspended mate- rial clogs the filter and prolongs filtration, it may be necessary to increase the diameter of the filter or decrease the sample volume. To obtain an estimate of total suspended solids, calculate the dif-
ference between total dissolved solids and total solids.
(V 1-V2)*N*8000
COD mg/l = ----------------------------------
Calculation:
(A-B) X1000
Where,
Sample volume in ml
Total suspended solids mg/l = ---------------------------------- Sample volume in ml
V1 = volume of Fe (NH 4 ) 2 (SO4 ) 2 required for titration against the blank, in ml;
V2 = volume of Fe (NH 4 ) 2 (SO4 ) 2 required for titration against the sample, in ml;
N = Normality of Fe (NH 4) 2 (SO4 )2 ;
V0 = volume of sample taken for testing, in ml.
4) Total Dissolved Solids
The filtrate remain in beaker after filtering the sample through filter contain dissolved solids. It
Includes mainly inorganic salts small amount of organic matter and dissolved gases.
Where, A = weight of filter + dried residue, mg, and
B = weight of filter, mg.
6) Mixed Liquor Volatile Suspended Solids (MLVSS) :
MLVSS or Mixed Liquor Volatile Suspended Solids is a test for the amount of volatile suspended solids found in a sample of mixed liquor. Volatile solids are those solids which are burnt up when a sample is heated to 550°C. Most of the volatile solids in a sample of mixed liquor will consist of microorganisms and organ-
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1137
ISSN 2229-5518
ic matter. As a result, the volatile solids concentration of mixed liquor is approximately equal to the amount of microorganisms in the water and can be used to determine whether there are enough microorganisms present to digest the sludge.In a wastewater treatment plant, operators should test for MLSS three times per week and for MLVSS once per week. Both tests should use grab samples taken from the same location in the treatment plant.
• Collect a grab sample of mixed liquor.
• Measure the total suspended solids in your sample will proba- bly need to use a smaller sample volume, such as 5 mL. Rec- ord the sample volume and the combined sample and filter weight in the Data section. At least 10% of all samples should be analyzed in duplicate.
• Ignite the filter and the total suspended solids residue from step 1 in a muffle furnace at 550°C. An ignition time of 15 to
20 minutes is usually sufficient for 200 mg residue. However,
when igniting more than one sample or when igniting heavier samples, the ignition time may need to be increased.
• Let the filter cool partially in the air until most of the heat has dissipated. Then transfer the filter to a dessicator to cool the rest of the way to air temperature.
• Weigh the filter and record the weight in the Data section.
• Repeat the cycle of igniting, cooling, desiccating, and weigh- ing until a constant weight is obtained or until the weight change is less than 4% or 0.5 mg, whichever is less.
7).Sludge Volume Index (sludge settleability)
The sludge volume index (SVI) is the volume in millilitres oc- cupied by 1 g of a suspension after 30 min settling. SVI typically is used to monitor settling characteristics of activated sludge and other biological suspensions. Although SVI is not supported the- oretically, experience has shown it to be useful in routine process control. Sludge volume index (SVI) is an indication of the sludge settleability in the final clarifier. It is a useful test that indicates changes in the sludge settling characteristics and quality.
By definition, the SVI is the volume of settled sludge in millili- tres occupied by 1 gram of dry sludge solids after 30 minutes of settling in a 1000 ml graduated cylinder or a settleometer. A liter of mix liquor sample is collected at or near the outlet of the aera- tion tank, settled for 30 minutes in a 1 liter graduated cylinder, and the volume occupied by the sludge is reported in millilitres. The SVI is computed by dividing the result of the settling test in ml/liter by the MLSS concentration in mg/L in the aeration tank times 1000
Settled sludge volume (ml/l) X 1000
SVI= --------------------------------------------- Suspended solids (mg/l)
V.CONCLUSION
The activated sludge methods of waste water treatment are the most economical and widely used for removing organic compo- nents from waste water. The pollution load was estimated by Chemical Oxygen Demand (COD).
In this project effort was made to determine the feasibility of activated sludge process (ASP) for the treatment of sugar industry wastewater and textile industry wastewater ,to develop simple
design criteria under local conditions. A bench scale model com- prising of an aeration tank and final clarifier was used for this purpose. The model was operated continuously for 30 days. Set- tled distillery wastewater was used as influent to the aeration tank. Chemical Oxygen Demand (COD) of the influent and efflu- ent were measured to find process efficiency at various mixed liquor volatile suspended solids (MLVSS) and hydraulic detention time (θ).
Thus the sugar industry effluent which is untreated exhibits high COD, BOD, TDS, contents and low contents of DO which is toxic to plants, so it is not permissible for irrigation. Treated effluent of sugar industry which is well balanced of chemicals if it is diluted with other fresh water, will be suitable for irrigation purposes. The treated effluents of sugar industry are not highly polluted and they satisfy the BIS Indian standard values. . On the basis of the present study, suitable treatment technology can be devel- oped for the treatment of sugar industry wastewater and textile wastewater in continuous mode of operation which shall have advantage to treat large quantity of effluent in shorter duration. The study can be used for the treatment of different types of in- dustrial waste water and the COD reduction, which will meet to the standard discharge value of COD, set by Maharashtra Pollu- tion Control Board (MPCB). Based upon this research, further work is proposed to study the nitrogen removal in addition to BOD5 and COD in ASP. In addition, effect of different MLVSS concentration and detention time on the efficiency of settling tank may be investigated.
VI. REFERENCES
1. B.Shah, R.Tailor, and A.Shah,”Adoptation of baggase fly ash sugar industry solid into zeolitie material for the uptake of phenol” Env.progess and sustainable Energy 30(3), 2011, 358-
367.
2. M.Ahamaruzzaman, “Role of fly ash in Removal of organic pollutants from wastewater”, Energy Fuels, 23 (3), 2009,
1494–1511.
3. S.G. Reznik, I. Katz, C.G.Dosoretz, “Removal of dissolved or- ganic matter by granular-activated carbon adsorption as a pre- treatment to reverse osmosis of membrane bioreactor efflu- ents”, Water Research, 42(4), 2008, 1595-1605.
4. P S. Marin and R. Beiras “Adsorption of different types of dis- solved organic matter to marine phytoplankton and implica- tions for phytoplankton growth and Pb bioavailability”, J. Plankton Res., 33(9), 2011, 1396-1409.
5. A.Matilainen, M. Vepsalainen, and M. Sillanpaa “Natural or- ganic matter removal by coagulation during drinking water treatment: A Review”, Adv. in Colloid and Interface Sci.,
159(2), 2010, 189-197.
6. M.L. Hami, M.A. Al-Hashimi, and M.M. Al-Doori, “Effect of activated carbon on BOD and COD removal in a dissolved air flotation unit treating refinery wastewater”, Desalination,
216(1-3), 2007, 116- 122.
7. P.M.Álvarez, J.P.Pocostales, and F.J. Beltran “Granular acti- vated carbon promoted ozonation of a food-processing sec- ondary effluent”, J. Hazard. Materials, 185(2-3), 2011, 776-
783.
8. P. K.Chaudhari, I. M.Mishra, and S. Chand “Decolourization and removal of chemical oxygen demand (COD) with energy recovery: Treatment of bio digester effluent of a molasses-
based alcohol distillery using inorganic coagulants”, Colloids
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1138
ISSN 2229-5518
and Surfaces Physicochemical. Eng. Aspects, 296(1-3), 2007,
238-247.
9. S. Mohana, B.K.Acharya, and D.Madamwar “Distillery spent wash: Treatment technologies and potential applications – A Review”, J. Hazard. Materials, 163(1), 2009, 12-25.
10. Y. Satyawali, and M. Balakrishnan, “Wastewater treatment in
molasses-based alcohol distilleries for COD and colour re- moval-A review”, J. Environ. Mgmt., 86(3), 2008, 481-497.
11. H.E. Reader and W.L. Miller, “Effect of estimations of ultra- violet absorption spectra of chromophoric dissolved organic matter on the uncertainty of photochemical production calcula- tions”, J. Geophysical Research, 116(9), 2011, 1029-1039.
12. H. Hyung and J.H. Kim, “Natural organic matter (NOM) ad- sorption to multi-walled carbon nanotubes: effect of NOM characteristics and water quality parameters”, Environmental Science and Technology, 42(12), 2008, 4416-4421.
13. E. B. Besselievne, and M. Schwartz, “The Treatment of Indus- trial Waste” 2nd ed., McGraw Hill Koga Kusha Ltd, 1976.
14. Metcalf and Eddy “Wastewater Engineering, Treatment and
Reuse” 4th ed., Tata McGraw Hill, 2003.
15. P.N.Modi “Sewage Treatment and Disposal and Wastewater
Engineering” Standard Book house Delhi, 1st ed., 280-300,
2001.
16. N. Musee, M.A. Trerise, and L. Lorenzen, “Post-treatment of Distillery Wastewater after UASB using Aerobic Techniques”, S. Afr. J. Enol. Vitic, 28(1), 2007, 50-53.
17. S. K. Gupta, and G. Singh “Anaerobic Hybrid Reactor – A promising Technology for the Treatment of Distillery Spent Wash”, J. Indian School of Mines, 11(1), 2007, 25-38 Borja,R
Martin A Maestra R Lugue M and Duran J1993 enhancement of Anaerobic digestion of wine distillery wastewater by re- moval of phenolic inhibitors Bioresour Technol 45,99-104.
18. Borja, R, Martin A Maestra ,R Lugue M and Duran J1993 en- hancement of Anaerobic digestion of wine distillery wastewater by removal of phenolic inhibitors Bioresour Tech- nol 45,99-104.
19. Coetzee G., Malandra L., Wolfaaradt G.M.and Viljoen Bloom M.2004 Dynamics of microbial bio film in rotating biological contractor for the treatment of winery effluent. Water SA30 (3)407-712.
20. Natraj ,S.K.Hosmani ,K.M.and Aminabhavi T.M.2006”Distillary waste water treatment by the membrane based nanofiltration based on nanofiltration and reverse osmo- sis”, water Res 40, 2349-2356.
IJSER © 2014 http://www.ijser.org
