International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 381
ISSN 2229-5518
Study of morphology and Zeta Potential analyzer for the Silver Nanoparticles
Mohammed J. Haider* , Mohammed S. Mehdi
Abstract
This work was devoted for production of colloidal silver nanoparticles via chemical reduction method. The colloidal silver nanoparticles were characterized using Transmission Electron Microscopy (TEM) and Zeta potential. The controllability of particle size and size distribution is shown in this paper to be dependent upon the type of measurement conditions in addition to it proved that the smaller mean diameter at average electric field (-
14.42 V/cm) and average current (-0.06 mA). The obtained particles were spherical in shape and having an average particles size of 5-20 nm, zeta
potentials range between -25.5 to -38.3 mV. The dissolution data indicates that the release of the silver nanoparticles is inversely correlated with the size of the nanoparticles i.e. the release increased with smaller size particles. The results showed that the Ag NPs would be stable in the pharmaceutical preparations and will be easily to the infection site. The colloidal silver nanoparticles were found to be very efficient antibacterial agents for different types of bacteria. The results provided strong evidence that could warrant the consideration of silver nanoparticles as antibacterial and antifungal agent.
Keywords: Silver nanoparticles, Zeta-potential, antibacterial, antifungal, chemical reduction methods
—————————— ——————————
1 INTRODUCTION
The properties and behavior of materials at the nano-scale or level vary greatly when compared to micro levels. Thus, many researches focus on the nobel metal Nanoparticles because of their unique properties which are different from those of bulk materials. These properties depend on their size, shape and differences in the environments of nanoparticles [1]. The synthesis of noble metals nanoparticles attracts an increasing interest due to their new and different characteristics as compared with those of macroscopic phase, that allow attractive applications in various fields such as medicine, biotechnology, optics, microelectronics, catalysis, information storage and energy conversion [2], antibacterial activity [7] and combine with another materials (like MWCNTs) to form new hybrid material with the diameter of one nanotube about a few nanometers (10 to 25 nm) and the average size of the highly dispersed AgNPs is estimated to be about (11-
80) nm [8]. Thus different preparation methods produce silver nanoparticles with different sizes and shapes. The fabrication technique has great influence on the properties of nanoparticles because of the plasmonic and antimicrobial properties of silver nanoparticles (Ag NPs particles exhibit remarkably unusual
physicochemical properties and biological activities) [3, 4].
Also Silver nanoparticles have the properties of a high surface area, very small size (<20 nm) and high dispersion. Moreover, colloidal silver solutions (CSSs) have an increased interest due to their antimicrobial properties with direct applications in pharmacology, veterinary medicine and so on. The interaction of metal nanoparticles with microorganisms (from fungi to viruses, e.g. HIV) is an expanding field of research. It is quite believed that the mechanism of the antibacterial effect of silver nanoparticles involve their absorption and accumulation by bacterial cells and accompanying shrinkage of the cytoplasm membrane or its detachment from the cell wall. Alternatively, silver ions might interact with the S–H bounds of the proteins which is leading to the inactivation of them. Upon binding of the nanoparticls to DNA molecules, (DNA molecules become condensed and lose their ability to replicate) which may be the main mechanism by which the nanoparticles inhibit the bacterial replications with. Understanding the forces governing the colloidal stabilities [5] is an essential requirement for the preparation of colloidal nanoparticles. Particles can be stabilized either electrostatically or sterically. Sterical stabilization is accomplished by adsorbing
either polymers or surfactants onto the surfaces of the particles to
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 382
ISSN 2229-5518
overcome nanoparticles agglomeration [6]. While, the electrostatic stabilization is attained by capping with charged molecules which form chemical bonds with/or chemisorbs onto the particles [3]. Silver powder was believed to have a beneficial healing effect for ulcers.
Over the last decade, there have been a great amount of studies
regarding toxic effects of Ag NPs on cells and micro-organisms. Although the cytotoxicity mechanisms behind the activity of Ag NPs on cells and bacteria are still not well understood, the three most common mechanisms have been proposed; (1) uptake of free silver ions to disrupt ATP production, (2) generation of reactive oxygen species by Ag+ and AgNPs and (3) direct damage to cell membrane by AgNPs attack [4].

In this paper we focus on the preparation and study the characterization of silver nanoparticle. The goal of this work is to demonstrate the effects of zeta potential analyzer on nucleation, growth mechanisms, stability and size distribution. Changes in the conditions of measurements used in laser ablation provide a simple and flexible technique to modify the properties of nanoparticles.
*Email: mohemedjumaa@yahoo.com
Laser and Optoelectronic engineering department, university of technology, Baghdad, Iraq
2 Experimental work
The experimental work of this research includes various methods for preparation Ag NPs:
2.1 Preparation of Citrate-AgNPs.
Raw-MWCNTs (0.1 g) with Purity (:> 95wt%, outside diameter: 5-
15 nm, Inside diameter: 3-5 nm and Length: ~30µm /USA) were dispersed in mixture of concentrated sulfuric acid 95% H 2 SO4 and nitric acid 65%HNO3 (3:1) under ultrasonication technique for 30 min to produce oxidized carbon nanotubes (MWCNT-COOH) . The samples were washed with deionized water (D.I) and dried at
70°C for 24 h.
2.2 Preparation of PVP-AgNPs.
PVP-AgNPs was prepared by the seed-mediated method.16 9 mL of 0.1 M silver nitrate was added to 280 mL of 5.36 g/L aqueous PVP suspension while stirring. After stirring for 5 min, 11 mL of
0.08 M sodium borohydride was then added to the solution, and stirred for 10 min. The PVP-AgNPs were ultracentrifuged at
14,000 rpm, then re-suspended in water.
3. Characterization of Silver Nanoparticles
3-1 Zeta Potential Measurements
Zeta potential is a physical property which is given the net surface charge of the nanoparticles, when these particles inside the solution repelling each other’s since produced Coulomb explosion between the charges of the nanoparticles giving rise to no tendency for the particles to agglomerate. The criteria of stability of NPs are measured when the values of zeta potential ranged from higher than +30 mV to lower than -30 mV [5]. Surface zeta potentials were measured using the laser zeta meter (Malvern zeta seizer 2000, Malvern). Liquid samples of the nanoparticles (5ml) were diluted with double distilled water (50 mL) using NaCl as suspending electrolyte solution (2 x10-2 M NaCl). The pH was then adjusted to the required value. The samples were shaken for 30 minutes. After shaking, the equilibrium pH was recorded and the zeta potential of the metallic particles was measured. A zeta potential was used to determine the surface potential of the silver nanoparticles. In each case, an average of three separate measurements was reported. The criteria of stability of NPs are measured when the values of zeta potential ranged from higher than +30 mV to lower than -30 mV [6].
3.2 Transmission Electron Microscopy (TEM)
The size and morphology of the silver was studied via TEM (Jeol, Japan). The microscope was operating at an accelerating voltage of 80 kV. The silver samples were first diluted (1:10) in distilled
water, and an aliquot (20 µL) was applied onto a carbon coated
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 383
ISSN 2229-5518
grid. The solution was then left for 1 minute and the excess was removed from the grid by blotting with a filter paper .The grids were placed in the grid box for two hours to dry before imaging.
4 Results and discussion
Measurements of zeta potential were also carried out in order to study the stability of nanoparticles as this extremely important for many applications, Surface zeta potentials were measured using the zeta analyzer (Malvern Zs – Zetasizer) Liquid samples of the nanoparticles (5ml) were diluted with double distilled water (50 mL) and the pH was then adjusted to the required value. The samples were shaken for 30 minutes. After shaking the zeta potential of the metallic particles was measured. A zeta potential was used to determine the surface potential of the silver nanoparticles. In each method, an average of two separate measurements was reported.
Table 2 is summarizing the zeta potential measurements of samples in a solution form. For synthesis nanoparticles using different methods, zeta values were measured and found to be -
53.18 mV at pH=7. The value of the zeta potential of method one using citrate coated AgNPs provides satisfactory evidence about their little tendency towards aggregation when its negative charges with a diameter of 71.6nm. This behavior unambiguously suggests the presence of strong electric charges on the particle surfaces to hinder agglomeration. These values were found to fall in the negative side which showed the efficiency of the capping materials in stabilizing the nanoparticles by providing intensive negative charges that keep all the particles away from each other. This result suggests that the Ag NPs particles and thus their solution is stable which is also in accordance with the result reported before for colloidal nanoparticles dispersion behavior as shown in Fig (1).
While the value of zeta potential in method two using The PVP- AgNPs is a positive charge with diameter about 119 nm which indicate that the tendency for aggregation or formation of self
assembled aggregates. Spontaneously, aggregation could have occurred due to frequent collisions between neighboring particles.
This feature for a positive charge of AgNPs might be due to the co-existence of dispersed silver ions. Ag+ ion was attached on the AgNPs and revealed the screening effect of the surface charge as shown in Fig (2).
Table 2: Z-Potential analyzer of used materials
N o | | Sample Mean Diam Zeta Mobility Conductivity Diamet eter potential er (nm) (mV) (cm²/Vs) (mS/cm) (nm) |
| Sample Mean Diam Zeta Mobility Conductivity Diamet eter potential er (nm) (mV) (cm²/Vs) (mS/cm) (nm) |
1 Method - 1.8 71.6 -53.18 -4.147e-004 0.1372 1 |
2 Method- 2.3 119.0 33.78 2.634e-004 0.0435 2 |
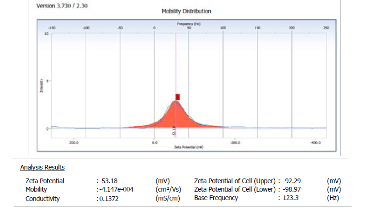
Fig (1): show the volume distribution and zeta potential using method one
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 384
ISSN 2229-5518
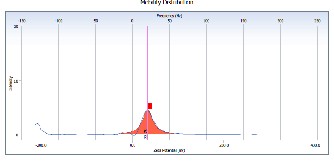

Fig (2): show the volume distribution and zeta potential using method two.
Table 1 and Figs 1 and 2 are summarizing the zeta potential measurements of samples in a solution form at different values of PH ranges. For the obtained nanoparticles, zeta values were measured and found to fall between -53.18 and 33.78 mV. These values provide full stabilization of the nanoparticles, which may be the main reason in producing particle sizes with a narrow size distribution index. Values of the zeta potentials of the citrate treated silver nanoparticles in addition to their narrow size distributions provide satisfactory evidence about their little tendency towards aggregation. This behavior unambiguously suggests the presence of strong electric charges on the particle surfaces to hinder agglomeration. These values were found to fall in the negative side which showed the efficiency of the capping materials in stabilizing the nanoparticles by providing intensive negative charges that keep all the particles away from Reddick threshold .Charged particles are able to interact across long distances via electrostatic forces and the surface charge may be disrupted as a way to induce nanoparticles assembly by modifying the condition of the reaction.
In this work, colloidal silver nanoparticles were proven stable over a wide range of the pH with no tendency for aggregation or formation of self assembled aggregates. This observation is contrary to suggestions that surface modification is mandatory to
the particles . It universally accepted that charged particles are
capable of interacting across a long distance scale via electrostatic repulsive forces. Disruption of the surface charges plays a paramount role in the assembly of the nanoparticles. Therefore, using these nanoparticles as a drug may provide a new horizon in avoiding bacterial and fungal resistance. In the studied series the electric charges were strong enough to hinder agglomeration and to provide stabilization of the nanoparticles. That suggests that the Ag NPs particles and thus their solution is stable which is also in accordance with the result reported before for colloidal nanoparticles stability.
Figs (2,a,b) and (3,a,b) show the size and the morphology of the used method one and tow. They were studied using transmission electron microscopy (TEM). The morphology and the diameters of the particles were measured. The silver particles in the citrate coated AgNPs and PVP-AgNPs were in a spherical form with a well defined particle size. The particle size strongly depend preparation conditions. The average particle size of the measured particles was as small as 5 to 50nm.

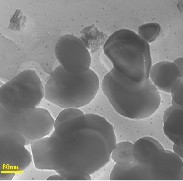
Fig 2: TEM micrographs of prepared AgNPs using sodium citrate a)Low and b) high magnifications
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 385
ISSN 2229-5518
5. Conclusions
This work suggests the silver nanoparticles particle of the size ranging from 5-50 nm and their solutions are stable at different values of pH range. The distribution of particles size of silver became narrower and mono distributed upon capping them with citrate. The dissolution data indicates that the release of the silver nanoparticles is inversely correlated with the size of the nanoparticles i.e. the release was increased with smaller size particles. The results proved that the Ag NPs would be stable in the pharmaceutical preparations and will be easily to the infection site. The nanoparticles show high anti-bacterial and antifungal effect. Also this work concludes preparation various AgNPs with different surface charges. Morphological changes of the vesicle after addition of x-AgNPs was different depending on the coating materials and surface charge of x-AgNPs.
References
[1] V. Piriyawong, V. Thongpool , P. Asanithi, P. Limsuwan, Effect of Laser Pulse Energy on the Formation of Alumina Nanoparticles Synthesized by Laser Ablation in Water , Surface Science Direct, 32 (2012): 1107-1112.
[2] V. Thomas Mathew, K. Sunny ," Studies on the antimicrobial properties of colloidal silver nanoparticles stabilized by bovine serum albumin", Colloids and Surfaces B: Biointerfaces 101 (2013):14– 18
[3] Salem H F, Eid KAM, Sharaf , Formulation and evaluation
of silver nanoparticles as antibacterial and antifungal
agents with a minimal cytotoxic effect, International
Journal of Drug Delivery 3 (2011):293-304.
[4] Ha Nee Umh and Younghun Kim, Simple Analysis for Interaction between Nanoparticles and Fluorescence Vesicle as a Biomimetic Cell for Toxicological Studies, Bull. Korean Chem. Soc. 2012, Vol. 33, No. 12.
[5] Y. Zhang, M. Yang, N.G. Portney, D. Cui, G. Budak, E.
Ozbay, M. Ozkan, C.S. Ozkan, Zeta Potential: A Surface Electrical Characteristic to Probe the Interaction of Nanoparticles with Normal and Cancer Human Breast Epithelial Cells, Biomed. Microdevices, 10 (2008):321–328.
[6] E. Akman, B. Genc Oztoprak, M. Gunes, E. Kacar, A.
Demir, Effect of Femtosecond Ti:Sapphire Laser Wavelengths on Plasmonic Behaviour and Size Evolution of Silver Nanoparticles, Photon Nanostruct: Fundam Appl., 9 (2011):276–286.
[7] J. Adawiya Haider, R. Mohammed M., A. Emad Jaffar Al- Mulla, S. Duha Ahmed," Synthesis of silver nanoparticles decorated Carbon Nanotubes and its antimicrobial activity against growth of Bacteria", Rend. Fis. Acc. Lincei ,2014
[8] J. Adawiya Haider, R. Mohammed M., S. Duha Ahmed," Preparation and characterization of multi walled carbon naotubes /Ag nanoparticles hybrid materials", International Journal of Scientific & Engineering Research, Vol.5 (3) 2014.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014
386
ISSN 2229-5518
386
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014
387
ISSN 2229-5518
387




