International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 1746
ISSN 2229-5518
Study of chemical interactions between modified starches and starches and cement
GBAGUIDI Victor S., TCHEHOUALI Adolphe, FANOU S. Ghislain, SANYA Emile, TOGNONMEGNI François
Abstract— This work has been done in order to valorize starch in industrial mortars. Its effect on cement hydration has been studied to propose the mechanism of interaction. After the characterization of raw materials, the study of cement hydration in dilute environment (W /C=20) enables us to propose mechanisms between polysaccharides and cement. Electrical conductivity measurement combined with the pH one and ion chromatography revealed that in the cement environment, the dextrose and the heated native cassava starch disrupt release of sulfate ions and consumption of calcium ions by adsorbing on first hydrates, slowing or blocking then the hydration of cement.
Index Terms— Starch, polysaccharides, chemical interactions, conductivity, chromatography, ions.
—————————— ——————————
1 INTRODUCTION
o face competitiveness in construction, increased aesthetic demands of architects and new constraints on the envi-
a) For conductivity and salinity studies, we used a conduc- timeter type WTW 340i (see Fig. 1.).
ronment and safety at work, concrete with additives has become a technical and economic necessity. Starch is a poly- saccharide extracted from plants. Its molecular formula is (C6H10O5) n and it is made of routes α (1-4) between several molecules of glucose.
Research on the interactions between polysaccharides in- cluding starch (modified or not) and the cement is still incom- plete. Several studies have focused on the interaction with monosaccharide (Luke Thomas) and the interaction with poly- saccharides such as: Cellulose Ether, Ether Starch, Starch Na- tive, Modified Starch (Peschard A., L. Bertrand); but these studies have not explored all types of polysaccharides and all aspects of interaction.
Our study aims at helping to elucidate chemical mecha- nisms between native and modified starches and cement.
We also used:

Fig. 1. Conductimeter
2 MATERIALS AND METHODS
2.1 Materials
Three polysaccharides were used:
- A native starch extracted from sweet cassava noted AN.
- The dextrose noted D.
- The cement used for the studies is Portland of type CPJ35
noted C.
On one hand, the chemical interactions have been studied
through the electric conductivity and salinity of cement sus-
pensions with or without additions; on the other hand, the
study by ion chromatography has been done to appreciate
influences on ions concentrations in cement midst.
————————————————
• GBAGUIDI Victor S. is Professor Master of lectures, Department of Civil Engineering, Polytechique School of Abomey-Calavi, University of Abomey- Calavi, Bénin, 071 BP 291 COTONOU. E-mail: gbagvict@yahoo.fr
• TCHEHOUALI Adolphe is Doctor, Associate Master of lectures, Department of Civil Engineering, Polytechique School of Abomey-Calavi, University of Abomey-Calavi, Bénin, 03 BP 3620 COTONOU. E-mail: tchehoua@yahoo.fr
- An electronic balance
- A cup
- A graduated cylinder
- A clean duster
b) Moreover, the study by ion chromatography was per- formed with a Dionex IC 1000 type connected to a computer for data recording (see Fig. 2.);

Fig. 2. Ion chromatograph, type Dionex IC1000 connected to a computer
For ion chromatography study, we also used:
- Thirty small plastic bottles
- The pipettes of 0 - 1 ml and 1 - 10mL
- A 1 mL an 10mL capacity syringe
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 1747
ISSN 2229-5518
2.2 Methods
a) Ion chromatography
During operations, the samples were taken from a thermo stated at 25 ° C chamber and kept under constant agitation.
The studies were undertaken in a diluted environment
(W/C=20) and the content in additive was fixed at 4%.
Samples of two (2) mL are made every hour. Then, each one
is filtered and diluted 10 times; 1 ml of the diluted solution is
injected into the ion chromatograph for analysis. During the
circulation of the solution through the circuit of the chromato-
graph, a curve (chromatogram) is gradually drawn on a screen
and the concentration of the major ions present in solution can
be read. The test lasts fifteen (15) minutes per sample.
b) Electricity conductivity
The electrode of conductimeter is cleaned with distilled water. Then, we use a clean cloth to wipe thoroughly until the device points out 0 (zero) µS/cm (micro siemens / cm).
The electrode was then immersed in the experimental solu- tion and we wait for about thirty seconds until the value dis- played on the dial stabilizes. Finally, we note the value of the electrical conductivity.
c) The salinity
The salinity can be calculated from the electrical conductivity measurement. To get the salinity S from electrical conductivity C, we use the following formula: S=0.00048×C.
With: C= conductivity in µS/cm and S= salinity in mg/kg.
3 RESULTS
3.1 Influence of the starches on the electric conductivity and salinity
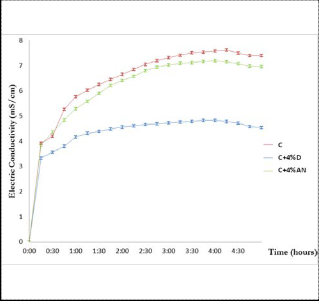
After periodic measurements of the electrical conductivity of the different cement compositions, the results are:
Fig. 3. Effects of starches on electric conductivity
The curves have a maximum value around 4:00. Firstly, there is a sudden increase in the conductivity with the three curves. This increase corresponds to the cement constituents’ dissolu- tion step. Note also that the initial value of the conductivity is slightly lower for the composition C + Dextrose. This shows action of dextrose on the dissolution of the cement's composi- tions.
Then, we note that the conductivity slowly increases in the compositions C and C + NA while stagnating in mixture C + Dextrose. So there is germination of first hydrates (CSH and ettringite) in C and C + AN while there is inactivity in compo- sition with dextrose. We can conclude that in addition to its action on the dissolution, dextrose prevents the formation of first hydrates.
Finally, there is a drop in conductivity in C and C + AN af- ter 4:00. This step reflects the precipitation of portlandite (CH) and makes us conclude that native starch does not prevent the precipitation of portlandite (CH).
Dextrose added to the cement to 4% significantly reduces the electrical conductivity of the medium of cement. It delays the initial dissolution of the cement components (less ions), while the same dose of native starch has virtually no effect on the evolution of the electrical conductivity of cement suspen- sions.
Salinity and electrical conductivity are related by a constant
(0.48/1000) we obtain the same trends for the study of salinity.
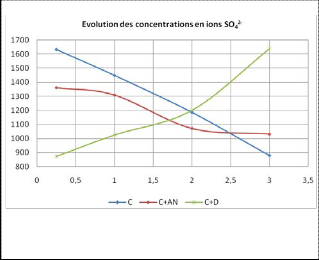
3.2 Influence of the starches on the evolution of the concentration in ions S0 2-
Fig.4. Evolution of [S042-] in the presence of the starches
During the first hours, 4% native starch does not have a great influence on the evolution of the concentration of sulfate ions.
In the case of dextrose, and at the same dose, the initial concentration of S04 2- ions is smaller than the control mixture. Then, it increases to the initial concentration we obtained in the reference mixture after dissolution. So, dextrose slows formation and consumption of these ions in the cement midst confirming the finding made from the conductometric study.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 1748
ISSN 2229-5518
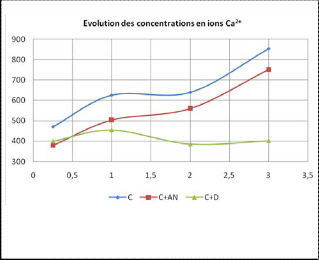
3.3 Influence of the starches on the evolution of the concentration in ions Ca2+
Fig. 5. Evolution of [Ca2+] in the presence of the starches
As we noticed for S042- ions, during the first hours, 4% native starch does not have a great influence on the evolution of the con- centration of sulfate ions; It reduces the amount of calcium ions produced;
At the same dose, the dextrose virtually blocks the formation of these ions; these results confirm what we concluded from con- ductivity and salinity studies.
4 CONCLUSION
The study shows that in diluted environment, dextrose could serve as set-retarding; it reduces the electrical conductivity of cement admixture, slows the consumption of sulfates ions and increases calcium ions concentration.
Dextrose is retarder; it reduces the electric conductivity of the cement suspensions and they slow down the consumption of the ions sulfates as well as they increase in the concentrations in ions calcium. The native starch practically does not produce effects.
REFERENCES
[1] A. Govin, “Aspects physico-chimiques de l’interaction bois- ciment/Modification de l’hydratation du ciment par le bois,” PhD dis- sertation, ENSM-SE, Jean Monnet Univ., Saint-Etienne, France, 2004.
[2] A. Peschard, “Aspect physico-chimiques de l’interaction ciment- polysaccharides dans les enduits/ Effet des polysaccharides sur l’hydratation du ciment,” PhD dissertation, ENSM-SE, Jean Monnet Univ., Saint-Etienne, France, 2003.
[3] G.M. Glenn, “Lightweight Concrete Containing an Alkaline Re-
sistant Starch-Based Aquagel,” Journal of Polymers and the Environ-
ment, 2004
[4] P. Grosseau, “Impact of cellulose ethers on the C3S hydration,” In-
ternational Congress on the Chemistry of Cement, Montréal, Canada,
2007.
[5] D.C. Okpala, “Cassava and maize starches as admixtures in con- crete,” Journal of the Institution of Engineers. India. Civil Engineering Di- vision, pp. 169-176, 1991
[6] P. Mounanga, “Experimental study of the behavior of cement pastes at the very youth: hydration, withdrawals, properties thermophysical,” PhD dissertation, Nantes Univ., France, 2003.
[7] T. Ferguson, “Tutorial : Mesurer des températures par thermocouples,”
National Instruments Corporation France, Note d’application 043, available at
http:// www.ni.com, Juil. 2009.
[8] Y. Baguma and M. Kawuki, “La filière industrielle du manioc dans les pays ACP : un mythe ou une option raisonnable,” National Crops Re- sources Research Institute, P.O. Box 7084, Kampala, Ouganda., 2006.
IJSER © 2013 http://www.ijser.org




