International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 68
ISSN 2229-5518
Simulation and Processing Techniques of Surface Electromyography Signal to Control a Lower Extremity Exoskeleton
Ngoc-Khoat Nguyen and Thi Mai Phuong Dao
—————————— ——————————
URFACE electromyography signal – based control is one of the successful and efficient control methods for a lower extremity exoskeleton robot. Many researchers have been considering deeply this technique. Most of them attempted sEMG signal which directly reflects human muscle activity as one of the most important control signals for their systems [7], [8], [10], [11]. Raw sEMG signals which are recorded by sur- face electrodes, however, are not being used by many re- searches because of certain drawbacks, such as very small and stochastic amplitude, unstable shape, and many noises [12]. Although sEMG signals can be measured carefully, their arti- facts often occur to destroy and make them almost useless. As a result, it is necessary to process these raw signals to create significant control signals by applying sEMG processing tech-
niques [1], [2], [12].
For the start point of this work, the first phase of this paper
introduces three techniques to simulate sEMG signal. These
techniques are based on three sEMG models: random variable
model, layer volume conduction model, and fiber action po-
tential model. Simulation results of these models are obtained
by using MatLab software. Three of these results illustrate clearly sEMG signals of Gastrocnemius Medialis, Tibialis An- terior, and Soleus muscles during human gait cycle which will significantly impact on our ongoing design of a lower extremi-
ty exoskeleton system.
In the second phase, a sEMG signal processing technique
will be considered. Continuous wavelet transform (CWT) is
used efficiently to analyze and remove noises from the raw
sEMG signal which has many drawbacks as mentioned above.
In order to generate control signals such as forces [7], [14], [16]
————————————————
• Thi Mai Phuong Dao is currently pursuing PhD degree program in Automa-
tion in Hunan University, China. At present, she is also a lecturer in Hanoi
University of Industry, Hanoi, Vietnam. Phone - +8615200931744. E-mail:
bkblackrose@yahoo.com
in accordance with sEMG signals, a sEMG-to-force relationship is given. Results of this processing technique are analyzed by both simulation and experiment during human gait cycle.
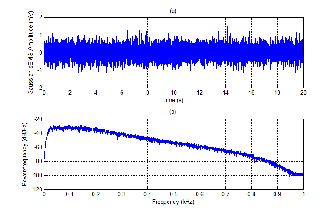
SEMG simulation techniques are very plentiful and they de- pend on actual aims. For simple goals, sEMG signal can be simulated as a random distribution [17]. To yield this work, a pseudo – Gaussian distribution with the roughly equal mean of zero is used. Fig. 1 presents the graph of sEMG which is plotted as a pseudo – Gaussian distribution. This simulation technique is also used to analyze the power spectrum density (PSD) of sEMG signal.
Fig. 1. Simulation of the Gaussian sEMG signal
(a) Shape of the Gaussian sEMG signal
(b) Power Spectrum Density of the Gaussian sEMG signal
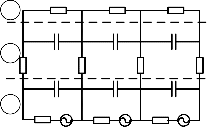
In order to understand the relationship between physiolog- ical and electrical models, a layer volume conduction model is used as shown in Fig. 2. According to this model, three layers of a physiological model (skin, fat tissue, and muscle fiber lay- er) are substituted by components of an electrical model. Val- ues of resistors and capacitors are computed by [13], [20] to simulate sEMG signal.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 69
ISSN 2229-5518
1

1
2
3
2
5 4 RFT
Rs
CFT
CM
RFT
Rs
CFT
CM
RFT
Rs
CFT
CM
RFT
Complex controller
sEMG Processing
Command signal
sEMG EXO-assisted signal Human
Human sensor
signals
Lower extremity Exoskeleton
Sensor system
3 RM V1
RM V2
RM V3
Sensor signal based control
Fig. 2. Layer volume conduction model
1-Skin, 2 – Fat Tissue, 3 – Muscle Fiber, 4 – Motor Unit, 5 – Bone
r – radius of motor unit
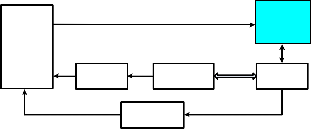
Fig. 4. A normal sEMG-based control scheme for a lower extremity exoskeleton system
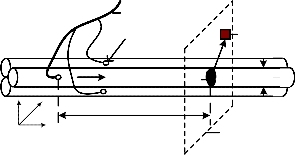
VMUAP (t) which can be obtained by using surface electrodes. In order to create a real sEMG signal, EMG(t), the additive noise, n(t), is considered as given in (4)
Axon
EMG(t) = VMUAP
(t) + n(t)
(4)
Muscle fibers
y x
z
Motor
endplates r
Excitation s z = vt
Electrode dm
Plane - z of the electrode
where, n(t) is chosen as a white noise for our work.
The most important characteristic of sEMG signals is to reflect directly the human muscle activities [12]. As a result, they can be used to create main input signals such as force or torque to control a lower extremity exoskeleton robot in accordance with the user’s motion intention. With their drawbacks as
Fig. 3. A fiber action potential model
To illustrate the extracellular action potential which is ob- tained by using surface electrodes, a fiber action potential model is considered as presented in Fig. 3. The intracellular action potential, VF (z), in mV, is computed by (1)
96(λ z)3 exp(−λ z) − 90, if z > 0 ;
mentioned earlier, however, they have to be processed before using as input control signals.
Fig. 4 introduces a control scheme for a lower extremity exoskeleton robot. The signal processing block contains some steps which are used to process sEMG signal as expressed be- low:
(a) After acquisition, raw sEMG signals should be ampli-
V (z) =
0,
(1)
otherwise
fied with suitable gains.
(b) Amplified sEMG signals will be modified by using
where, z, in mm, is the distance along the fiber and λ, in mm-1, is a scale factor which can be defined by experiments [9]. By calculating the second derivative of VF (z), the equation of transmembrane current is yielded
d 2 (V (z))
rectification or Root Mean Square (RMS) methods.
(c) Modified sEMG signals will be filtered by applying a
FIR low pass filter with a 2-10 Hz cut-off frequency
[17].
In the first step, to remove bias, it is necessary to calculate the
I F (z) = KI
dz2
(2)
mean of whole sEMG signals and then subtract it from each
data point. In the second step, a full-wave rectification method
where, KI is a proportionality constant [9], [18]. To obtain re- sults in time domain, z is substituted by v.t, where v is the propagation velocity of the action potential and t is the time value. According to E.V. Stalberg et al in [18], this velocity can be calculated by using the value of the fiber diameter dm.
The single fiber action potential, VAP (z), in mm, can be yielded by (3)
+∞ ∂V (z)∂(1/ r)
is usually used to obtain the absolute values of sEMG signals. The other method which uses the definition of RMS is applied successfully in many researches [1], [12] because it gives a sig- nificant measure of the signal power.
In order to remove noises from sEMG signals to get the lin- ear envelope of the signal as mentioned in the third step, a low pass filter can be used. In our work, to generate a good de- noised sEMG signal, the continuous wavelet transform (CWT)
VAP (z) = KV ∫ dS ∫
![]()
F dz
∂z2
(3)
will be used. The definition of CWT [3], [4] is expressed in (5)
S −∞
+∞
where, KV is a proportionality constant [19], and r is the dis-![]()
CWT (a,b) = 1
![]()
∫ EMG(t).w t − b dt
(5)
tance between the fiber section S and the observation point
[19]. Other papers calculated the above potential by using the
a −∞
a
convolution between the transmembrane current and the weighting function [9], [18]. The total of the single fiber action potentials is used to compute the motor unit action potential
where EMG(t) is the analyzed signal, w(t) is a mother wavelet
function, b is a translation index, and a is scale parameter [5].
By applying CWT, noises in sEMG signal can be removed suc-
cessfully because the signal energy only concentrates into few-
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 70
ISSN 2229-5518
er coefficients while noise energy does not.
Though the amplitude of the processed sEMG signal at any
instant in time is random, it is roughly proportional to the
force exerted [1], [12], [21]. In this investigation, an EMG-to-
force function is used to calculate corresponding force F(A, B)
[21]
human gait cycles. These simulation results have more signifi- cance to control our lower extremity exoskeleton which assists humans during normal walking with or without load carry- ing.
To apply a signal processing technique, the real sEMG sig- nal of Gastrocnemius Medialis muscle is collected during a
F ( A, B) = A *[1 − exp(−B * EMG(t))]
(6)
human gait cycle by our experiment. The raw sEMG signal in
where EMG(t) denotes the processed signal and A, B are two muscle parameters. These parameters belong to the type of muscle and they can be defined by experiments. For the calcu- lation of sEMG-to-force, a normalization with the peak of Max- imum Voluntary Contraction (MVC) is normally used [12], [14] to compare sEMG readings between subjects.
Although sEMG signals belong to many parameters, in our work, we only concentrate on simulation results in time do- main concerning our experiment results. The intracellular ac- tion potential and the transmembrane current are plotted in Fig. 5(a) and Fig. 5(b), respectively. In Fig. 5(a), algebraic signs of the first derivative of V(z) affect the orientation of the di- poles: depolarization with the positive sign and repolarization with the negative sign. Fig. 5(b) expresses different shapes of the transmembrane current with different values of the fiber diameter: dmin , dmax , dTibialis Anterior , and dGastrocnemius Medialis . These values can be found well in [19]. To understand more clearly about the effect of the fiber diameter, Fig. 6 shows in- tracellular action potentials which belong to the change of this parameter. According to this relationship, minimum and max- imum values of the intracellular action potential are shown stably, however, corresponding times are changeable.
To calculate the singly extracellular action potential, we can use either the definition of convolution or applying (3). Fig. 7 presents simulation results of an extracellular action potential by calculating the convolution of the transmembrane current and the weighting function. This weighting function is defined by J. Duchene et al in [9]. Algorithms of Fast Fourier Transform (FFT) and Inverse Fast Fourier Transform (IFFT) are also used to compute this extracellular action potential as signal train in Fig. 7(b).
By applying (4), sEMG signal and its PSD can be formed as presented in Fig. 8. Here, P.D.Welch’s method is used to ex- press the PSD of sEMG signal by using the corresponding function in MatLab software. This simulation result is similar in comparison with the Gaussian sEMG signal in Fig. 1. The shape of the simulated sEMG signal is clearly appropriate in accordance with the real signal.
In order to match with experiment results, three lower muscles of human leg are considered during the gait cycle. A human gait cycle is normally divided into two phases: stance phase and swing phase. These phases will be divided into sev- en smaller phases which can be found clearly in [22]. Three concerned human lower muscles are Gastrocnemius Medialis, Soleus, and Tibialis Anterior. The timing of the muscle activa- tion and the intensity of muscle contraction in accordance with the different gait phases can be found in [19]. Simulation re- sults of these lower muscles are shown in Fig. 9 during three
Fig. 10(a) will be processed by using Signal Processing Toolbox
in Matlab software version 2011b. First, any DC offset will be
removed from the raw sEMG signal, and then this signal is
rectified by applying a full-wave rectification as shown in Fig.
10(b). Here, we can also use the RMS method to obtain abso- lute values of sEMG signal as plotted in Fig. 11(a). This abso- lute signal shape is similar to the rectified signal in Fig. 10(b). This rectified signal is then filtered by applying a low pass
filter of a 10Hz cut-off frequency with the zero-phase digital filtering. Here, we also use the sampling frequency of 1000Hz and the 5th order filter. The filtered sEMG signal is shown in Fig. 11(b). In order to create the corresponding force, the EMG- to-force relationship in (6) is used. As a result, the obtained force in Fig. 11(c) can be used to generate the command signal as mentioned earlier in the control scheme for a lower extremi- ty exoskeleton robot.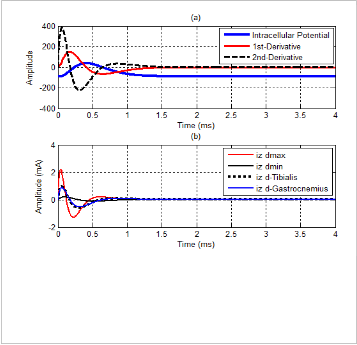
In this paper, the wavelet transform is also used to process sEMG signal by applying Wavelet Toolbox Main Menu in MatLab software. Normally, this technique can be used as a filter. For our work, it is applied to remove noises from modi- fied sEMG signal after using RMS method. Result of this pro- cessing is expressed in Fig. 12. Wavelet function of Daubechies db3, level 5 which has a matched shape with the motor action unit potential is used for our work. Fig. 13 shows more infor- mation of residuals for this processing, such as histogram, cumulative histogram, autocorrelations, and FFT-spectrum. According to these simulation results, most of the noises are removed effectively from sEMG signal to create the command signal more exactly.
Fig. 5. The intracellular action potential and the transmembrane current
(a) The intracellular action potential and its derivatives
(b) The transmembrane current belongs to change of fober di-
ameter: dmin = 25µm, dmax = 85 µm, dTibialis = 57 µm and dGas- trocnemius = 54 µm.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 71
ISSN 2229-5518
This paper presents simulation and processing techniques of sEMG signal as the first part of our work in which a sEMG – based control scheme for a lower extremity exoskeleton robot has been designed successfully. Simulation results obtained play a significant role in accordance with our experiments, especially, in design of our system to assist soldiers who wear exoskeleton robots to work under hard conditions. By apply- ing improved signal processing techniques, sEMG signal re- cording can be used efficiently to create the corresponding force which will be made the command signal for our exoskel- eton system. For our work in the near future, we will develop strongly these techniques to design an exoskeleton – assisted system which can be applied widely in our real life.
Fig. 6. The intracellular action potential belongs to the change of the fiber diameter.
(a) Intracellular action potentials of Tibialis anterior mus-
cle, dmin, and dmax.
(b) Change of intracellular action potentials from dmin =
25µm, up to dmax = 85 µm.
Fig. 9. Simulation of sEMG signal during 3 gait cycles
(a) Gastrocnemius Medialis
(b) Tibialis Anterior
(c) Soleus
Fig. 7. A single fiber and motor unit action potential
(a) A single muscle fiber action potential which is calculated by the convolution of the transmembrane current and the weighting function.
(b) The motor unit action potential
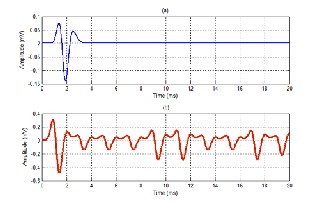
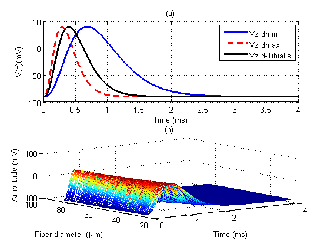
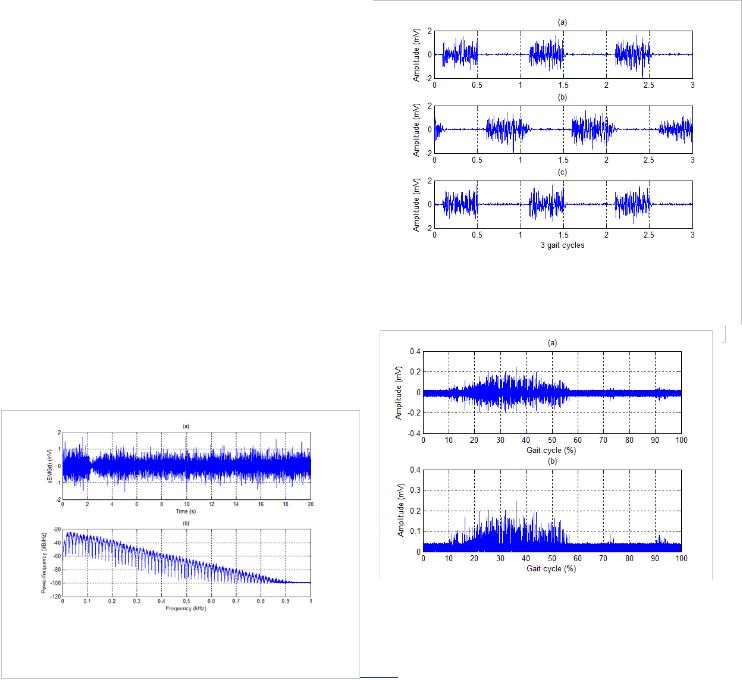
Fig. 8. Simulation of sEMG signal and its PSD (a) sEMG signal with noises
(b) PSD of sEMG signal
Fig. 10. Real sEMG signal from Gastrocnemius medialis during gait cycle
(a) Raw signal
(b) Rectified signal
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 72
ISSN 2229-5518
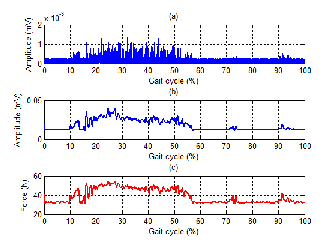
Fig. 11. The processed sEMG signal and the corresponding force
(a) RMS sEMG signal
(b) Filtered signal
(c) The corresponding force
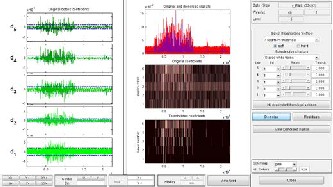
Fig. 12. De-noised sEMG signal by applying wavelet transform
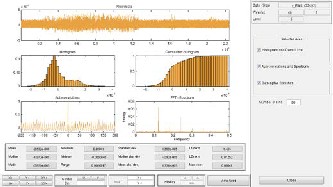
Fig. 13. More on residuals for wavelet transform to make the de- noised sEMG signal
The authors wish to thank members of PRMI laboratory for their support to perform this exoskeleton project.
[1] Christian Fleicher, Konstantin Kondak, Christian Renicke, and Guiter Hommel, “Online Calibration of the EMG to Force Relationship”, Proc. IEEE/RSJ. International Conference on Intelligent Robots and Sys- tems, pp. 1305-1310, Sept. 28 - Oct. 2, 2004.
[2] Claudia U. Ranniger and David L. Akin, “EMG Mean Power Fre-
quency Determination Using Wavelet Analysis,” Proc. IEEE/EMBS.
19th International Conference, pp. 1589-1592, Oct. 30- Nov. 2, 1997.
[3] Ali N. Akansu, Wouter A.Serdijin, and Ivan W. Selesnick, “Emerging
Applications of Wavelets: A Review”, Physical Communication, pp. 1-
18, 2010.
[4] Oliver Rioul and Martin Vetteru, “Wavelets and Signal Processing,” IEEE SP Magazine, pp. 14-35, Oct. 1991.
[5] Qi Xu, Yazhi Quan, Lei Yang, and Jiping He, “An Adaptive Algorithm for the Determination of the Onset and Offset of Muscle Contraction by EMG Signal Processing,” IEEE Trans. Neural Systems and Rehabilitation Engineering, vol. 21, no. 1, pp. 65-73, Jan. 2013.
[6] A.K. Mahabalagini, K. Ahmed and F.Schlereth, "A Novel Approach for Simulation, Measurement and Representation of Surface EMG Signals", Conference Record of the Forty Fifth Asilomar Conference on.
ASILOMAR 2011: Signals, Systems and Computers, pp. 476-480,
2011.
[7] Ram Murat Singh and S. Chatterji, "Trends and Challenges in EMG Based Control Scheme of Exoskeleton Robots - A Review." International Journal of Scientific and Engineering Research, vol. 3, no.8, pp. 1-8, 2012.
[8] Gregory S. Sawicki and Daniel P. Ferris, "Mechanics and Energetics of Incline Walking with Robotic Ankle Exoskeletons", Journal of Experimental Biology, vol. 212, no.1,pp. 32-41, 2009.
[9] J. Duchene and J.-Y. Hogrel, "A Model of EMG Generation", IEEE Trans. Biomedical Engineering, vol. 47, no.2, pp. 192-201, 2000.
[10] Kazuo Kiguchi and Y. Imada, "EMG-based Control for Lower-Limb Power-Assist Exoskeletons", IEEE Workshop on. RIISS '09: Robotic Intelligence in Informationally Structured Space, pp. 19-24, 2009.
[11] H.He and K. Kiguchi, "A Study on EMG-based Control of Exoskeleton Robots for Human Lower-Limb Motion Assist", 6th International Special Topic Conference on. ITAB 2007: Information Technology Application in Biomedicine, pp. 292-295, 2007.
[12] Konral, Peter, The ABC of EMG, A Practical Introduction to
Kinesiological Electromyography, pp.30-55, 2005.
[13] Fansan Zhu and N. W. Levin, "An Electrical Resistivity Model of Segmental Body Composition Using Bioimpedance Analysis", Proceedings of the 25th Annual International Conference of the IEEE. Engineering in Medicine and Biology Society, pp. 2679-2682, 2003.
[14] D.P. Ferris and C.L. Lewis, "Robotic Lower Limb Exoskeleton Using Proportional Myoelectric Control", Annual International Conference of the IEEE. Engineering in Medicine and Biology Society, pp. 2119-
2124, 2009.
[15] Dario Farina and Roberto. Merletti, "A Novel Approach for Precise
Simulation of the EMG Signal Detected by Surface Electrodes", IEEE Trans. Biomedical Engineering, pp. 637-646, 2001.
[16] O. Unluhisarcikli, M. Pietrusinski, B. Weinberg, P. Bonato, and C.
Mavroidis, "Design and Control of a Robotic Lower Extremity Exoskeleton for Gait Rehabilitation", 2011 IEEE/RSJ International Conference on. Intelligent Robots and Systems (IROS), pp. 4893-4898,
2011.
[17] S. Patrick, J. Meklenburg, S. Jung, Y. Mendelson, and E.A. Clancy, "An Electromyogram Simulator for Myoelectric Prosthesis Testing", Proceedings of the 2010 IEEE 36th Annual Northeast. Bioengineering Conference,pp. 237-243, 2010.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 73
ISSN 2229-5518
[18] Sanjeev D.Nandedkar, E.V. Stalberg and Donald B.Sanders, "Simulation Techiques in Electromyography", IEEE Trans. Biomedical Engineering, vol. 32, no. 10, pp. 775-785, 1985.
[19] W. Wang, A. De. Stefano, and R.Allen, "A Simulation Model of the Surface EMG Signal for Analysis of Muscle Activity During the Gait Cycle", Computer in Biology and Medicine, vol. 36, no. 6, pp. 601-618,
2006.
[20] A. Salman, J. Iqbal, U. Izhar, U.S. Khan, and N. Rashid, "Optimized Circuit for EMG Signal Processing", 2012 International Conference on. Robotics and Artificial Intelligence (ICRAI), pp. 208-213, 2012.
[21] Mitsuhiro Hayashibe, David Guiraud, and Philippe Poignet, “EMG- to-Force Estimation with Full-Scale Physiology Based Muscle Mod- el”, Proc. IEEE/RSJ. International Conference on Intelligent Robots and Systems, pp. 1621-1626, Oct. 11-15, 2009.
[22] Chetas D. Joshi, Uttama Lahiri, and Nitish V. Thakor, “Classification of Gait Phases from Lower Limb EMG: Application to Exoskeleton Orthotics,” 2013 IEEE Point-of-Care Healthcare Technologies, pp. 228-
231, Jan. 2013.
IJSER © 2013 http://www.ijser.org