
International Journal of Scientific & Engineering Research Volume 4, Issue 1, January-2013 1
ISSN 2229-5518
Smitha Sudhakar & Dr. A.Jayakumaran Nair
Abstract: Marek’s disease virus, a gamma herpes virus is the causative agent of Marek’s disease affecting poultry industry. Vaccination strategies against the disease have resulted in tremendous evolutionary pressure which forced the virus to emerge into new pathotypes. Herpes virus of Turkey’s, a serotype -3 virus of the Marek’s family has been the most commonly used vaccine world-wide. In this research three hypothetically immunogenic envelope glycoproteins, viz. gC, gD and gI of HVT (FC126) were identified, amplified, analysed and expressed in pKLAC yeast expression system for the first time.The identity and specificity of the recombinant glycoproteins were tested and confirmed with an aim to analyse the immunological potential of these glycoproteins in field animals
Index Terms: Marek’s Disease,Herpes virus,Glycoproteins,Vaccines, Pathotypes,Chicken,Immunity
In our present study we have cloned and expressed the glycoprotein genes gC, gD and gI of HVT FC126 in pKLAC expression vector. These proteins are highly cell associated [11] and until now they have not been expressed invitro [13]. Once expressed in a suitable system, these glycoproteins may be used to challenge field chicken to analyse their immunogenic effect.The first step was the propagation of virus done using chorio-allantoic membrane (CAM) method in specific pathogen-free eggs (12day old) [3], [18].
-------------------------------------------------------------------------------
1. Smitha Sudhakar is currently research scholar at The Department of Biotechnology, University of Kerala,India.
2.Dr.Jayakumaran Nair is currently Professor at The Department of Biotechnology,University of
Kerala,India
The virus was multiplied in primary Chick embryo fibroblasts which were infected 4 h after seeding with 0.5 plaque-forming units of HVT per cell and incubated for 72h. When a cytopathic effect was apparent to 80% of the cells, the virus was isolated and DNA was separated using 0.5M EDTA – Proteinase K method [18]. The HVT DNA was observed as a single 23kb intense band on 0.6% agarose gel and compared with Hind III λ digest DNA ladder [Fig1.1].
The PCR primers were designed in accordance with the HVT genome sequence published in NCBI GenBank and according to K.lactis Protein Expression kit manual. The forward primers gC, gD and gI contained an Xho1 restriction site and a Kex protease cleavage site immediately followed by the first codon of the gene’s ORF and the reverse primers included a Not1 site followed by the stop codon [Table1.1]. PCR was performed according to the conditions [8], [Table 1.2]. The amplification products were analyzed on 1.6% agarose gel and were found to be a single intense band of ~ I.5 kb,1.1kb and 1.0 kb for gC, gD and gI respectively [Fig 1.2].The amplicons were purified using Axyprep PCR purification kit.
A yeast vector pKLAC2 has been used for the secreted expression of glycoproteins of HVT in Kluyveromyces lactis (Yeast). PCR products and pKLAC2 were digested with Notl and Xhol enzymes and the digestion mixtures were purified using Genei™ gel extraction kit .The ligation of digested PCR product with linearized pKLAC2 was done and the ligation mixture was run on 1% agarose gel and compared with I kb ladder DNA to confirm ligation. The
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 1, January-2013 2
ISSN 2229-5518
ligated product was observed as a single band of expected size. Ecoli strain JM109 (end AI, recA I, gyrA96, laqlqZΔAM) cells were used for transformation of the glycoprotein genes as described in pKLAC instruction manual.
To confirm successful transformation PCR was done for the inserts with the following conditions - initial cell breakage and DNA denaturation for 5 minutes at 94°C followed by
35 cycles consisting of denaturation at 94°C for I minute, annealing at 55°C for 2 minutes, and extension at 72°C for
3minutes. As a final extension the reactions were held at
72°C for 10 minutes. The specificity of the PCR products were analysed by running on 1.6% agarose. Plasmid DNA was prepared by alkaline lysis method [8].The existence of insert DNA in the recombinant plasmid was also approved by restriction digestion analysis of the isolated plasmids from transformants. The purified plasmids were subjected to restriction digestion by Notl and Xhol, the digested products along with I kb ladder DNA were run on
1 % agarose gel containing ethidium bromide. Finally the
inserts were sequenced bidirectionally using sequence specific primers, compiled and aligned using NCBI nBLAST. The exact sequence similarity of the query with the reference confirmed successful cloning.
Later pKLAC2 containing gene of interest (gC, gD and gI) were linearized with SacII to generate an expression cassettes The digestion mixture was run on 1.1% agarose gel to give bands of 7.8kb, 7.4kb and 7.3kb respectively for gC, gD and gI respectively [Figure 1.3]. Transformation was done by introducing the linearized expression cassette into K.lactis cells using the Klactis GG799 competent cells and Yeast Transformation Reagent. Transformants were selected by growing cells on yeast carbon base (YCB) agar medium plates containing 5 mM acetamide. On incubation at 37oC, the transformed cells appeared on plates. The correct integration of expression cassette into the K.lactis genome was investigated by colony PCR of the positive clones and negative clones using integration primers to amplify a 2.4 kb product [Figure 1.4]. A single colony of cells which positive for integration of expression cassette were harvested by scraping with a sterile pipette tip and resuspended in 2 ml of YPGal media in a sterile culture tube. The culture was incubated with shaking (I80 rpm) at
30°C for 3-4 days toob obtain a saturated culture (culture density of > 30 OD 600 units/ml).1 ml of each culture was taken and centrifuged to pellet the cells and SDS-PAGE was as per Laemelli et al., 1970 with both to check induction of recombinant protein.
Polyclonal antibodies against MDV-I were raised in rabbits by hyper-immunizing two male New Zealand white rabbits. The presence of anti-MDV-l antibody in rabbit immune sera was analyzed by an enzyme linked immunosorbent assay. The hyper-irnrnune serum collected on 45thday post immunization showed maximum antibody titer against MDV-1. This serum was used for western blot to detect the recombinant glycoproteins C, D, I and Western blotting of the recombinant glycoproteins was done [12]. On Western blot, the recombinant glycoproteins C, D and I got bound with the primary antibody and prominent signals were visible showing bands of 42kd, 41kD and
40.5kD respectively for glycoproteins gD, gC and gI[Figure
1.5], indicating the authenticity of expressed recombinant proteins.
Glycolink immobilization kit was used to purify the recombinant glycoproteins by affinity chromatography. The Glycolink hydrazide activated resin coupled to oxidized sugar groups on the glycoproteins. Once purified the purity and specificity of the proteins were analysed by Western blot [Figure 1.6]. Although prokaryotic expression system such as E. coli is simple, it lacks the modification mechanism of eukaryotic expression. Post-translational modifications of eukaryotic systems, such as glycosylation, disulfide bond formation, and proteolytic processing, can be performed in yeast. Previous studies demonstrated that Kluyveromyces lactis is capable of expressing glycoproteins processed in a manner similar to virus infected insect cells. Both baculovirus and mamma- lian expression systems have generated recombinant proteins in eukarytoic cells, but they were not suitable for large-scale protein expression
.
Here protein secretion using pKLAC2 is achieved by generating a fusion between the HVT glycoprotein of interest and the α-MF secretion domain present in the vector. A gene or open reading frame of interest must be inserted into pKLAC2 so that it is in the same translational reading frame as the α-MF domain. It is also required to have a Kex protease processing site at the junction between the α-MF domain and the N-terminus of the protein of interest. Secretion resulted in production of glycoproteins that were significantly pure, that do not require difficult lysis of yeast cells to isolate, and that may have desired post-translational modifications that cytosolic proteins do not. Due to the presence of amdS gene in pKLAC2 only transformed cells can grow on YCB medium. Since YCB is nitrogen free medium, the product of the amdS gene acetamidase can utilize acetamide as a source of nitrogen
The HVT glycoproteins gC, gD and gI were expressed in a non-viral host for the first time. HVT is a highly cell
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 1, January-2013 3
ISSN 2229-5518
associated virus and hence the studies on the envelope glycoproteins is unwieldy until date. Here the highly cell associated glycoproteins have been mass produced in yeast cells and this finding has paved the way for further
research on the structural and functional aspects of these glycoproteins that are of prime importance in conferring immunity against MDV1 in chicken immunized with HVT.
gC Forward | 5' CTC GAG AAA AGA ATG ATT ATT GTC ACC ACT TCG 3' |
gC Reverse | 3' GCG GCC GCT CAT AGC CTG GTA TAC ACA TAC CGG C 5' |
gD Forward | 5 CTC GAG AAA AGA ATG CTT ATG ACT CCT ACA ATG 3' |
gD Reverse | 3' GCG GCC GCC TAT ACA ATT TCA TCA TCC GTC TC 5' |
gI Forward | 5' CTC GAG AAA AGA ATG GTT TCC AAC ATG CGC G 3' |
gI Reverse | 3' GCG GCC GCT TAA TTC CGC CCC GGT AGG TAA AAG 5’ |

IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 1, January-2013 4
ISSN 2229-5518
Cycle2 | Step1 Denaturation 94ºC for 1 minutes Step 2 Annealing 55ºC for 2 minutes Step 3 Extension 72ºC for 3 minutes Step 4 Go to Cycle 2 for 34 times |
Cycle 3 | Final Extension: 72ºC for 10 minutes |

Figure 1.1-Viral DNA Isolation Figure 1.2-PCR of Glycoprotein Genes
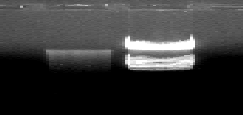
HVT DNA | HindIII λ | gC | gD | gI | DNA |
DNA digest | ladder |
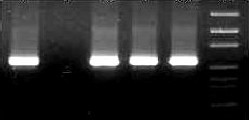
Figure 1.3- SacII Digestion of plasmids Figure 1.4- Colony PCR for r-plasmids

IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 1, January-2013 5
ISSN 2229-5518
gC gD DNA ladder gI Clone1 Negative Clone2 Clone3 Clone4 DNA Control ladder

Figure 1.5- Western Blot for GC,GD and GI Figure 1.6- SDS PAGE for GC,GD and GI

GD GC GI Marker GD GC GI
1. Beasley, J. N., Patterson, L .T and Mc Wade, D. H (1970). Transmission of Marek's disease poultry house dust and chicken dander. American Journal of Veterinary Research. 2:339-344.
2. Bulow, V. V., and Biggs, P. M. (1975).Differentiation between strains of Marek's disease virus and Turkey herpes virus by immunofluorescence assays. Avian Pathology. 4:133-146
3. Churchill, A. E, and Biggs, P. M. (1967). Agent of Marek's Disease in Tissue Culture. Nature, 215: 528-530
4. Churchill,A.E and Payne.L.N. (1969). Immunization against Marek's disease using a live attenuated virus. Nature, 221:
744-747
5. Ficken, M. D, Nasisse, M. P, Boggan, G. D, Guy. J. and Wages. D. P., Witter, R.L, Rosenberger, J. K, and Nordgren, R. M (1991). Marek's disease virus isolates with unusual tropism and virulence for ocular tissues: clinical findings, challenge studies and pathological features. Avian Pathology. 20:461-474.
6. Lubinski, J. M, Wang,L , Soulika, A. M, Burger, R. Wetsel, R. A., Colten.H, Cohen. G, H., Eisenberg, R. J, Lambris. J. D and Friedman, H. M (1998). Herpes simplex virus type I glycoprotein gC mediates immune evasion .Journal of Virology. 72:8257-826.
7. Rajcani, J., Vojvodova, A (1998).The role of herpes simplex virus glycoproteins in the virus replication cycle Acta
Virologica.42, 2. 103-118,
8. Sambrook , J., E, Fritsch. F, and T. Maniatis (1989). Molecular cloning: a laboratory manual, 2nd edition. Cold Spring
Harbor Laboratory, Cold Spring Harbor, N.Y. 2:202-20
9. Schat. K. A, Calnek B. W and Fabricant, J (1981). Influence of the bursa of fabricius on the pathogenesis of Marek's disease. Infection and Immunity. 31:199-207.
10. Smith, T. W, Albert. D. M., Robinson, N, Calnek. B W, Schwaec. O (1974). Ocular manifestations of Marek's disease.
Investigative Ophthalmology. 13:586-592.
11. Solomon, J. J, Witter. R. L, Nazerian, K.and Burmester, B. R (I968). Studies on the etiology of Marek's disease. I.
Propagation of the agent in cell culture. Proceedings of Society of Experimental Biology Med. 127:173-177
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 1, January-2013 6
ISSN 2229-5518
12. Towbin. H, Staehelin. T, Gordon. J (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A.76,9:4350-4354
13. Witter, R. L., Nazerian. K„ Purchasc, H. G., and Burgoyne,G.H (1970). Isolation from turkeys of a cell-associated herpesvirus antigenically related to Marek's disease virus. American Journal of Veterinary Research. 31:525-538.
14. Witter, R. L (1971). Marek's disease research-History and perspectives. Poultry Science. 50:333-342.
15. Witter, R.L (1997). Increased virulence of Marek's disease virus field isolates. Avian Dis. 41:149-163
16. Witter. R.L. (1998). Control strategies for Marek’s Disease: A perspective for the future, Poultry Science. 77:1197–1203
17. Witter. R.L. (2001). Protective efficacy of Marek’s vaccines. Current topics in microbiology and immunology.255:57–90
18. Wyn-Jones, A. P. and Kaaden, O. R (1979). Induction of virus-neutralizing antibody by glycoproteins isolated from chicken cells infected with a herpes virus of turkeys. Infection and Immunity. 25:54-59
IJSER © 2013 http://www.ijser.org
IJSER 2013
http://www.ijserorq