Lecturer of department of Chemistry
Shri shakti degree college
Email : neelima2010.669@rediffmail.com
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 150
ISSN 2229-5518
Ms. Neelima,
Lecturer of department of Chemistry
Shri shakti degree college
Email : neelima2010.669@rediffmail.com![]()
The grafting of acrylonitrile (AN) onto drumstick mucilage was carried out in aqueous medium by ceric ammonium nitrate(CAN) initiator and under a nitrogen gas atmosphere to produce (PAN) grafted mucilage.The maximum percentage of grafting (%G) was ascertained to be 72% at the following optimum condition 25 0 C reaction temperature, 2 hour reaction period. The formation of grafted copolymer was confirmed by using IR and XRD analytical technique.
![]()
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 151
ISSN 2229-5518
Copolymers are prepared by polymerizing a monomer in the presence of a fully formed polymer of another monomer.”Grafting results into retention of desirable properties of base polymer and incorporation of favorable properties of grafted polymer”.
Incorporation of specific functional groups onto polymers influences the physical, chemical, thermal, mechanical and rheological properties of materials. Copolymerization is done to enhance the properties and the utility of a system in various applications.
Graft copolymerization is considered for designing wide variety of new materials. Hybridization of natural polymers is great interest because of their application to biomedical and biodegradable materials. The improvement of natural polymers by grafting other monomers has been finding a large interest in the scientific world and industry due to combinational properties of both natural and synthetic polymers.
The incorporation of polyacrylates into biopolymers and, specifically, the grafting of acrylic monomers onto starch and starch like biopolymers result in combined properties such as biocompatibility, non-toxicity, and higher bioadhesion, which would confer attractive characteristics on the newly prepared composite materials.
Grafting of acrylic monomers onto biopolymers is usually performed to prepare materials with high absorbency for water. Incorporation of vinyl monomers onto the
backbone of natural polysaccharides helps in improving some original properties of
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 152
ISSN 2229-5518
polysaccharides and also allows the product copolymers to show novel functionality. Natural polymers, mainly polysaccharides, can be the most desirable flocculants for various waste water treatments, if their shelf life increased. Grafting has been utilized as an important technique for modifying the properties of a polymer.
This project incorporates the results of the synthesis of graft copolyners of polyacrylonitrile (PAN) on to drumstick mucilage.
The whole pods are reported contain, an isothiocynate and thicarbamates and O – [2’- hydroxy 3’-(2” heptenyloxy] – propylundecanoate and O-ethyl-4-[α-1- rhamnnosyloxy)-benzyl] carbamate, methyl –p- hydroxybenzoate and β-sistosterol. The pods designated as drumstick polysaccarides, the investigation of which reveled the presence of glactose, dextrose, xylose and sodium, potassium, magnesium, calcium salts of glucuronic acid, contrary to the definition of mucilage, the presence of dextrose was exception.
Modification of the seed gums and mucilages by grafting on to it water-soluble vinyl monomers has been reported to result into retention of desirable properties and incorporation of favorable properties. Grafting results into retention of desirable properties of base polymer and incorporation of favorable properties of grafted polymer. Synthetic polymers though are more efficient but their non biodegradability acting as a poison to the environment. One great advantage thus gained is the consequent reduced biodegradability because of drastic change in the original regular structure of natural polymers. It is also observed that grafting of shear degradable polymers on to the
rigid polysaccharide back bone provides fairly shear stable systems.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 153
ISSN 2229-5518


Moringa oleifera (Pods and tree)
Drumstick are a rich source of the polysaccharide galactomannan. They are also a source of saponins such as diosgenin, yamogenin, gitogenin, tigogenin, and neotigogens. Other bioactive constituents of moringa include mucilage, volatile oils, and alkaloids such as choline and trigonelline.
Fourier Transform- Infrared (FT-IR) Spectroscopy,Model Make: Bruker, Vector 22 are useful for qualitative identification of molecular compounds.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 154
ISSN 2229-5518
Parameters.
aqueous solution of crude mucilage was prepared by continuous stirring with water for
12h at 60ºC and precipitating with saturated sodium hydroxide solution. The complex was separated by centrifugation and taken in 1M acetic acid stirred for 8h, centrifuged and precipitated with methanol. It was washed with 70, 80, 90 and 95% methanol. The precipitate was finally dried by keeping in oven at 40ºC for 24 hours.
Synthesis of Graft Copolymer of Moringa oliefera with Acrylonitrile were prepared (Ds-g-PAN): 0.5 gram of mucilage was dissolved in distilled water (150mL) in an Erlenmeyer flask. The flask was then sealed with septum stopper and nitrogen gas was flushed into the solution through hypodermic needle for 20 minutes. Then the required amount of acrylonitrile was added into the solution through the stopper by hypodermic syringe with constant stirring using magnetic stirrer. The solution was stirred for 30 minutes while being bubbled with nitrogen. The required amount of ceric ion solution (in 1N HNO3 ) was injected through the stopper by hypodermic syringe.
The nitrogen flushing was continued for another 20 minutes; then the needles were
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 155
ISSN 2229-5518
taken out, and the flask was further sealed with teflon tape. The reaction temperature was maintained at requisite temperature. The reaction mixture was stirred occasionally; the reaction was continued for required time and then terminated by injecting 0.5 mL of saturated aqueous hydroquinone solution.
The reaction product was precipitated in excess of isopropanol and filtered through sintered glass filter. The precipitate was again slurried in acetone followed by filtration and finally the precipitate was dried in vacuum oven at 40ºC. The homopolymer was extracted from the grafted product using acetone as solvent in a soxhelet extractor until a constant weight was obtained.
Effect of Initiator (CAN) Concentration: On increasing the concentration of initiator i.e., (CAN) from 0.5 × 10-3 moles to 1.5 × 10-3 moles, PG increased due to increase in the free radicals on polysaccharide chain. With further increase in (CAN) concentration, up to 2.50 × 10-3 moles, (PG) decreased. The falling of (PG) at higher (CAN) concentration is a well-known phenomenon and ascribed to the increasing participation of the ceric ion in the termination of the growing grafted chains.
radicals, resulting in decrease in (PG).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 156
ISSN 2229-5518
increase in temperature upto 500C. The decreased (PG) observed with increase in
temperature at 500C might be attributed to faster rate of termination and more homopolymerization at higher temperature.
Characterization of Graft Copolymers by IR: The FTIR spectra of pure mucilage and (Ds-g-PAN) (PG = 70%). The FTIR spectra of purified Moringa shows characteristic peaks of –OH between 3404-3150 cm-1, ether linkage at 1455-1400 cm-1,
-CH stretching between 2918 -2850 cm-1, -C-O stretching ether at 1059 cm-1, -C=O
stretching in aldehyde at 1632 cm-1, thioamide stretching 1245.66 cm-1 and -CH3 at
2923 cm-1. In the grafted sample of (Ds-g-PAN), the characteristic absorption at 2219 cm-1 of indicates grafting of (PAN) with drumstick. The O–H stretching frequency in the grafted product is broader as compared to that in spectrum of drumstick mucilage.
This is due to unequal hydrogen bonding in the grafted product again attributing to the
fact that grafting has occurred.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 157
ISSN 2229-5518
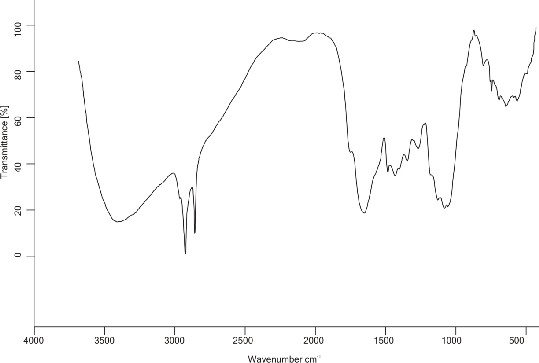
IR Spectrom of Drumstic mucilage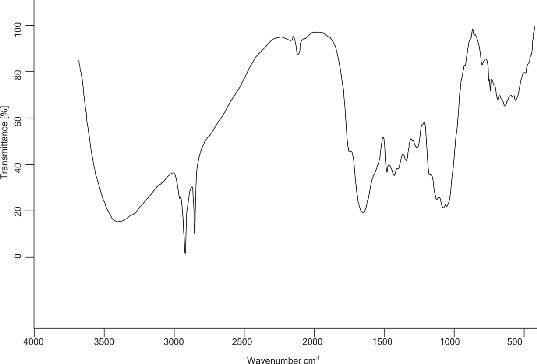
IR Spectrom of (Ds-g-PAN)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 158
ISSN 2229-5518
X-Ray Diffraction Analysis: XRD pattern of (Ds-g-PAN) supported grafting and revealed that pure mucilage is partially crystalline in nature, whereas after grafting, peaks for the crystalline areas appear near 2θ; 19.89º, 23.240 and 57.990, indicating the increased crystallinity on grafting due to grafted PAN chains on to the pure mucilage molecules .
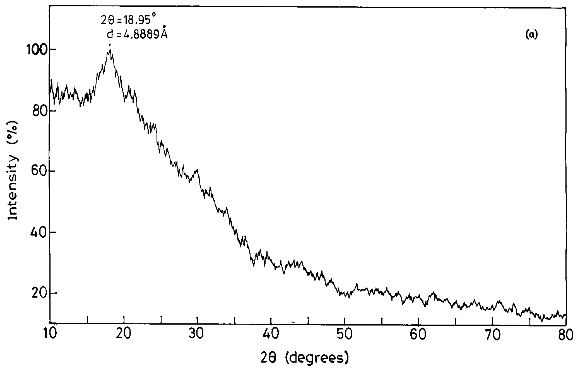
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 159
ISSN 2229-5518
The results of synthesis of polyacrylonitrile-grafted copolymers of drumstick mucilage. (Ds-g-PAN) was synthesized by grafting acrylonitrile (AN) onto purified drumstick mucilage by radical polymerization method in aqueous system using ceric ion/nitric acid redox initiator.
The following conclusions were drawn from present study “Synthesis of Copolymers of (PAN) and Moringa olifera mucilage “ The conclusions drawn proved that the objectives set before the study has been achieved to an appreciable extent.The variation in monomer concentration, initiator concentration, reaction time and temperature affected the percent grafting and grafting efficiency of the polysaccharides.
Gintert M J, Jana S C and Miller S G 2007 Polymer 48 4166
Huang C C, Jang G W, Chang K C, Hung W I and Yeh J M 2008 Polym. Int. 605
Jin H S and Chang J H 2008 J. Appl. Polym. Sci. 107 109
Njuguna J, Pielichowski K and Desai S 2008 Polym. Adv. Technol. 19 947
Mittal V 2007 J. Colloid. Interf. Sci. 314 141
Tjong S C and Ruan Y H 2008 J. Appl. Polym. Sci. 110 864
Wang C, Xue T, Dong B, Wang Z and Li H 2008 Wear 265 1923
Yang Y W, Chen Y T, Chen H C, Lin W T and Tsai C H 2009 Polymer 50 2856
Zhao F, Wan Y, Bao X B 2009 J. Colloid. Interf. Sci. 333 164
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 160
ISSN 2229-5518
Feng M, Chen Y, Gu L, He N, Bia J, Lin Y and Zhan H 2009 Eur. Polym. J. 45
Nataraj S K, Him B H, Yun J H and Lee D H 2009 Mater. Sci. Eng. B162 75
Sun D, Li Y, Zhang B and Pan X 2010 Compos. Sci. Technol. 70 981
Cai Z W 2010 Appl. Surf. Sci. 256 2490
Chen Y S, Chen C C and Hou S S 2010 J. Appl. Polym. Sci. 115 416
Karimi P, Rafizadeh M and Taromi F A 2011 J. Hazard. Mater. 186 182
Mansoori Y, Atghia S V, Zamanloo M R, Imanzadeh 2010 46 1844
Agudelo N A, Perez L D and Lopez B L 2011 Appl. Surf. Sci. 257,8581
IJSER © 2013 http://www.ijser.org