
Distillation was done in low flame. The left out seed residue was collected for experiment.
sInternational Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 106
ISSN 2229-5518
Removal of fluoride from water by Moringa oleifera
seed residue after oil extraction
Neeraj Agnihotri, Vinay Kumar Pathak, Naseema Khatoon , Masihur Rahman
Abstract— The search for new low cost materials is an important task of contemporary research in the environmental protection. Application of Moringa Oleifera as a natural coagulant has revealed efficient result for different pollutants removal. The present study deals with the use of Moringa Oleifera residue (MSR) powder as a coagulant for removal of fluoride and optimization of dose. The particle size of the respective coagulant particle was found to be (103.22±62.02 µm× 95±42.55 µm) which contains pores/cavities on the surface shows that average pore sizes is 6.01±3.23 nm × 7.26±3.33 nm in SEM image. Statistical analysis through two way ANOVA reveals that the removal efficiency of MSR coagulant found to be increased with decrease in initial fluoride concentration (2mg/l - 10mg/l) and increase in coagulant doses (4g/l – 18g/l). The optimum dose of coagulant was found to be 16g/l. The mean removal efficiency of 2mg/l (M=62, SD= 25.78) found to be significantly higher than other initial fluoride concentration (4mg/l-10mg/l). The maximum removal efficiency is found to be 95.22% for optimized dose.
Index Terms— Moringa Seed Residue (MSR), coagulant, fluoride, removal efficiency, ordinary two way ANNOVA, post hoc tukey HSD test, W HO
—————————— ——————————
LUORIDE in low concentration up to 1 mg/l in drinking water is useful in reduction of dental carries. But in higher concentration or dose due to repeated intake of high fluoride water leads to major health
issues such that fluorosis (dental, skeletal and soft tissue fluorosis) [1] .The WHO has set the guideline for fluoride in drinking water 1.5 mg/l [2]. Higher concentration of fluoride in India is due to the high fluoride containing minerals such as fluorspar, cryolite and fluorapatite in igneous and metamorphic rocks. The common methods used for the removal of fluoride from drinking water are precipitation (Alum, Lime, Alum + Lime , Gypsum + fluorite, Calcium choride); adsorption and ion-exchange (activated carbon, plant carbon, zeolites, defluoron 2, clay pots, activated alumina, bone, bone char); membrane filtration processes; distillation[3-5]. Study of removal of fluoride from various low cost biological materials like Rice husk, seed extracts of Moringa Oleifera, etc has already been established [6].
Application of Moringa oleifera (MO) as a natural coagulant has revealed efficient results for different pollutants removal [7-13]. Advantages such as less sludge generation, no pH variation throughout experiments and no need to pH adjustment have been reported for this natural coagulant [11].The present study deals with the development of novel method for removal of fluoride from Moringa oleifera seed residue after oil extraction. There is no
————————————————
Neeraj Agnihotri is pursuing Ph.D., Department of chemistry, Integral
University, Lucknow, India, .E-mail: agnihotrineeraj@in.com
Vinay Kumar Pathak is pursuing Ph.D. , Department of chemistry,
Integral University, Lucknow, India, .E-mail: vinay.vku@gmail.com
Naseema Khatoon is Associate Prof. , Department of chemistry, Integral
University, Lucknow, India, .E-mail: naseema615@yahoo.com
Masihur Rahman is Prof., Department of chemistry, Integral University,
literature available in which Moringa oleifera seed residue after oil extraction is used for fluoride removal.
All the chemicals used for the present study were of reagent grade (Fischer Scientific, India) and Milli-Q water (Millipore corp. with electrical resistivity less than 18 MΩ cm) was used for preparation of solution and dilution purpose throughout the experiment. Fluoride estimation was done by SPADNS method [14] using UV-Vis Spectrophotometer (LT-2800, Labtronics). ANOVA and graphical analysis was done by using GraphPad prism 6 software.
2.1 Preparation of Moringa Oleifera seeds
The dried fruit (brown coloured pods) of Moringa oleifera Lam (syn. M. pterygosperma) were collected from Lucknow, India. The seeds coat and wings were removed manually and grinded into a fine powder using a domestic blender and then sieving the ground powder through 250
μm sieves (Heico, HS 32.40).
2.2 Oil Extraction
Air-dried Moringa Oleifera seeds were crushed in to small pieces to increase the surface area of the kernel. The ground seeds are placed in a thimble and placed it in the sample compartment of the soxhlet extractor. The sample compartment was attached to a 500 ml round bottom flask containing 300-350ml hexane. Soxhlet set-up was assembled and heated in a mantle and soxhlet extraction was allowed to run for 3 hours. The hexane solvent was distilled out in the oil extract by rotatory evaporation.
Lucknow, India.
IJSER © 2013 http://www.ijser.org
sInternational Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 107
ISSN 2229-5518

Distillation was done in low flame. The left out seed residue was collected for experiment.
2.3 Preparation of Coagulant
The seed residue was dried for 2-3 days in hot air oven and later powdered with the help of house hold domestic grinder. Seed powder was sieved through 250 µm sieve (Heico, HS 32.40).
2.4 Characterization of MSR coagulant
To study of the surface area of coagulant SEM image are obtained. Sample (MSR powder) is mounted on Aluminium stubs with the help of double sided adhesive tape. The mounted stubs are coated with gold palladium (AuPd) alloy using POLARON sputter coater with 18 mA current and 2.4 KV voltage under nitrogen environment. The coated stubs are viewed under SEM LEO 430. Desired images are taken at magnification 500X and 1500 X shown in figure 1a and 1b respectively.
Figure .1a. SEM of MSR at magnification 500X showing MSR
coagulant particle of varying size
Figure .1b.SEM of MSR at magnification 1500x showing fibrous honey comb like highly porous structure.
Synthetic fluoride contaminated water for the jar tests was prepared by adding 22.1 mg of anhydrous sodium fluoride in 1000ml of milli Q water; 1.00ml=100µg F–. 100 ml of stock fluoride solution is diluted to 1000 ml to form a solution contains 1 ml = 10.0 µg F–.Other solutions are made by dilution of intermediate solution.
The widely used method to evaluate coagulation- flocculation processes is a jar test [15]. A standard jar test apparatus, the Phipps and Bird six-paddle stirrer with illuminated base was employed for the tests, with six 2-L square B-Ker2 Plexiglas jars. For each jar test, the following procedure was followed [16]. Each jar was filled with 2 L of sample measured with a graduated cylinder, and the initial temperature was recorded. The coagulant dose destined for each jar was carefully measured into 150-mL beakers, and distilled water was added to yield equal volumes in all the beakers. Mixing speed was set 150 rpm for 1 minute at the time of adding of coagulant and then 40 rpm for 20 minutes and a settling time of 30 minutes). Study on different pH had not been done because pH has no significant effect on fluoride removal by using protein coagulant found in Moringa Oleifera [14-15]. Coagulant dose was optimized using different coagulant doses i.e., 4g/l, 6g/l, 8g/l, 10g/l,
12g/l, 14g/l, 16g/l and 18g/l using initial fluoride
concentration of 10 mg/l. The effects of initial fluoride
concentration were analyzed for each fluoride concentration of 2mg/l, 4mg/l, 6mg/l, 8mg/l and 10mg/l. The % removal efficiency was calculated as:![]()
IJSER © 2013 http://www.ijser.org
sInternational Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 108
ISSN 2229-5518
Where, Co=Initial concentration and Ce=Final concentration of fluoride in mg/l.
All the experiments were done thrice and the average values of the result have been presented with minimum expected error of ±5%.
SEM image shows particle of varying size (103.22±62.02
µm× 95±42.55 µm). Highly magnified image at 1500X shows fibrous structure carries honey comb like pores or cavities on them. Further, analysis of pores/cavities on the surface shows that average pore sizes is 6.01±3.23 nm ×
7.26±3.33 nm.
The percentage removal of fluoride as a function of different coagulant doses (4g/l, 6g/l, 8g/l, 10g/l, 12g/l,
14g/l, 16g/l and 18g/l) at 28±50C for five different initial
concentration of fluoride (2mg/l, 4 mg/l, 6 mg/l, 8mg/l
higher dose i.e. 18g/l (M=77.09, SD=17.20) was significantly different than lower doses i.e., 4g/l(M=15.90, SD=6.31),
6g/l (M=27.14 , SD=8.44), 8g/l (M=33.83, SD=11.82), 10g/l
(M=40.67, SD=10.65), 12g/l (M=50.06, SD=13.16) and 14g/l
(M=65.78, SD=16.71). However dose 16g/l (M=75.83,
SD=18.30) did not significantly differ from dose 18g/l. Our
results suggest that beyond 16g/l coagulant dose there is very little or no increase in percentage of fluoride removal. Hence 16 g/l is required optimum coagulant dose. The stagnant or very little increase removal efficiency beyond optimum dose may be due to agglutination of coagulant particles at higher coagulant doses which leads to decrease in surface area and ultimately less and stagnant removal efficiency. The reason for removal efficiency of MSR powder is due to presence of coagulant protein in fiber as well as its fibrous, honey comb like structure. The suggested mechanism for coagulation and fluoride removal is suppose to be that positively charged protein bind to the part of surface of negatively charged fluoride ions through electrostatic attractions. This leads to the formation of positively and negatively charged areas of the particle surface. Due to particle collision and neutralization, formation of flocks with a net like structure take place which settle down due to the gravity.
and 10 mg/l) is shown in table 1.
TABLE1.0
![]()
Removal efficiency of variable doses of MSR as a coagulant for different initial concentration of fluoride
1 0 0
9 0
8 0
7 0
6 0
5 0
4 0
3 0
2 0
1 0
0
![]() 4 g / l
4 g / l ![]() 6 g / l
6 g / l ![]() 8 g / l
8 g / l ![]() 1 0 g / l
1 0 g / l ![]() 1 2 g / l
1 2 g / l ![]() 1 4 g / l
1 4 g / l
![]() 1 6 g / l
1 6 g / l
![]() 1 8 g / l
1 8 g / l
C o a g u l a n t d o s e i n g / l
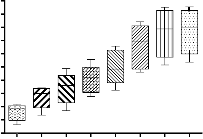
Figure 2 Box and whiskers plot diagram representing an ordinary two way ANOVA analysis between different coagulant doses to compare removal efficiency.
An ordinary two way ANOVA (Analysis of variance) between the different coagulant doses was conducted to compare the effect of coagulant doses for different initial concentrations of fluoride and determination of optimized dose of the coagulant for fluoride removal shown by box and whiskers plot in figure 2. There was a significant effect of higher coagulant doses on fluoride removal at p< 0.05. It is evident that removal of fluoride increases with increase in coagulant dose for 8 different coagulant doses [F (7.0, 28)
= 105.3, p<0.05]. Post hoc comparison using tukey HSD test
conditions indicated that mean removal efficiency for
The coagulating protein MO2.1 present in the MSR powder has molecular mass 6.5 kDa and isoelectric point is above pI10. A 3D structure of protein has eight positively charged amino acid (7 arginine and 1 histidine) and 15 glutamine residues [17]. The protein is thermo-resistant and hence also suitable for high average temperature (35-400C) in tropical countries.
Effect of initial concentration of fluoride by MSR coagulant had been studied for five different concentrations of fluoride i.e., 2mg/l, 4 mg/l, 6 mg/l, 8mg/l and 10 mg/l for different coagulant doses 4g/l, 6g/l, 8g/l, 10g/l, 12g/l,
14g/l, 16g/l and 18g/l at 28±50C and is shown in table 1.
IJSER © 2013 http://www.ijser.org
sInternational Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 109
ISSN 2229-5518
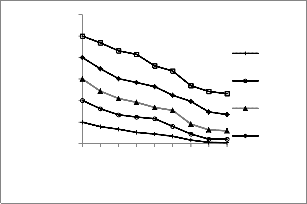
Observations were analyzed statistically using ordinary two way ANOVA (Analysis of variance) and is shown graphically by box and whiskers plot in figure 3. There is a significant role of initial fluoride concentration on removal efficiency (p< 0.05). It is evident that removal of fluoride increases with decrease in initial fluoride concentration among 5 different initial fluoride concentrations [F (4, 28 =
51.29, p<0.05]. The removal efficiency of any dose of
coagulant is to be found more for lower initial
concentration of fluoride might be because of abundant
positively charged binding sites available on coagulant to
bind with negatively charged fluoride ions. Post hoc comparison using tukey HSD test conditions indicated that mean removal efficiency of different dose coagulant for
12
10
8
6
4
2
0
0 4 6 8 10 12 14 16 18
Coagulant dose in g/l
2mg/l
4mg/l
6mg/l
8mg/l
lower initial concentration of fluoride i.e. 2mg/l (M=62.62, SD=25.78) was significantly higher than initial concentrations i.e., 4mg/l(M=56.43, SD=25.44), 6g/l
(M=50.75, SD=21.60), 8g/l (M=40.57, SD=17.61), 10g/l (M=31.03, SD=16.91). The maximum removal efficiency is found to be 95.49 % for coagulant dose18 g/l but not have any significance difference from optimum coagulant dose
16g/l i.e., 95.22%. There is significant decrease in the final
concentration of fluoride in synthetic sample after jar test for lower initial fluoride concentration and higher coagulant doses shown in figure 4.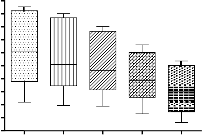
1 0 0
9 0
![]() 2 m g / l
2 m g / l
8 0
Figure 4 Graphical representation of final concentration of
fluoride at different coagulant doses in jar apparatus test (mixing speed150 rpm for 1 minute at the time of adding of coagulant and then 40 rpm for 20 minutes and a settling time of 30 minutes)
4 CONCLUSION
The result of present work showed effective removal of fluoride from Moringa seed residue (MSR) as a coagulant. MSR, obtained after oil extraction, is a solid waste and is generally disposed off but, it contains protein which has coagulant properties. MSR coagulant is found to be a potential coagulant for fluoride removal. The optimum dose of coagulant is found to be 16 g/l. The maximum removal efficiency is found to be 95.49 % for coagulant dose
18g/l and, 95.22%.for optimum dose 16g/l. The result![]()
7 0
![]()
6 0
5 0 ![]()
4 0 ![]()
3 0
2 0
1 0
0
2 4 6 8 1 0
I n i t i a l f l u o r i d e c o n c e n t r a t i o n i n m g / l
4 m g / l
6 m g / l
8 m g / l
1 0 m g / l
obtained reveals that MSR coagulant can play important role in removal of fluoride. The protein found in MSR coagulant is thermo-resistant, and this method of removal can be suitable for high average temperature (35-400C) in tropical countries. It is also having other advantages such as less sludge generation, no dependency on pH variation, low cost of production and environmental friendly. Future studies would be needed elucidate the practical use of MSR powder in removing fluoride and other contaminants in aqueous solution.
We are highly thankful to Dr P. K. Seth and Dr. Pragya Gupta from Biotech Park, Lucknow for providing us their valuable suggestions. We are also thankful to Birbal Sahni Institute of Palaeobotany, Lucknow for providing us the Scanning Electron Micrograph (SEM) image of the charcoal sample.
REFERENCES
[1] Susheel A, “Sound Planning and Implementation of fluoride and fluorosis mitigation Programme in an Endemic Village”, presented at the proceeding of international workshop on fluoride drinking water: Strategies, management and mitigation, Bhopal, 22-24 Jan 2001.
IJSER © 2013 http://www.ijser.org
sInternational Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 110
ISSN 2229-5518
[2] WHO (World Health Organisation), (2006). Fluoride in drinking water p.144. London, UK:IWA publishing.
[3] British Geological Survey (BGS), 2003. “Water Quality Fact
Sheet: Fluoride.” WaterAid International site.http://www.wateraid.org/documents/plugin_docume nts/fluoride1.pdf.pdf
[4] Heidweiller, V.M.L., 1992. “Fluoride Removal Methods. In
Endemic fluorosis in developing countries: causes, effects
and possible solutions” (Frencken, J.E., editor). Publication number 91.082, NIPG-TNO, Leiden, The Netherlands, 1992.
[5] Pickard, B. and Bari, M., 2004. “Feasibility of Water Treatment Technologies for Arsenic and Fluoride removal from groundwater.” AWWA Water Quality Technology Conference, San Antonio, Texas; November 2004. https://chppmwww.apgea.army.mil/dehe/pgm31/newpu bs/WQTC2004ArsenicProc(Pickard%2 and%20Bari).pdf
[6] C.M.Vivek Vardhan1 and J.Karthikeyan2 “Removal Of
Fluoride From Water Using Low-Cost Materials”, International Water Technology Journal, IWTJ Vol. I - Issue
2, September 2011
[7] Beltrán-Heredia, J., Sánchez-Martín, J., 2009. “Removal of sodium lauryl sulphate by coagulation/flocculation with Moringa oleifera seed extract”. J. Hazard. Mater. 164, 713-
719.
[8] Beltran-Heredia, J., Sanchez-Martin, J., Delgado-Regalado, A., 2009. “Removal of carmine indigo dye with Moringa oleifera seed extract”. Industrial & Engineering Chemistry Research 48, 6512-6520.
[9] Beltrán Heredia, J., Sánchez Martín, J., 2008. “Azo dye
removal by Moringa oleifera seed extract coagulation”. Coloration Technology 124, 310-317.
[10] Bhatia, S., Othman, Z., Ahmad, A.L., 2007. “Pretreatment of palm oil mill effluent (POME) using Moringa oleifera seeds as natural coagulant”. J. Hazard. Mater. 145, 120-126.
[11] Prasad, R.K., 2009. “Color removal from distillery spent wash through coagulation using Moringa oleifera seeds: Use of optimum response surface methodology”. J. Hazard. Mater. 165, 804-811.
[12] Pritchard, M., Craven, T., Mkandawire, T., Edmondson, A.S.,
O'Neill, J.G., 2010. “A comparison between Moringa oleifera and chemical coagulants in the purification of drinking water - An alternative sustainable solution for developing countries”. Phys. Chem. Earth 35, 798-805.
[13] Sharma, P., Kumari, P., Srivastava, M.M., Srivastava, S.,
2006. “Removal of cadmium from aqueous system by
shelled Moringa oleifera Lam. seed powder”. Bioresour.
Technol. 97, 299-305.
[14] APHA, AWWA, WPCF. Standard Methods for Examination
of Water and Wastewater, 21th edition, Washington, D.C, (2005).
[15] Kawamura, S. (1991) “Effectiveness of Natural Polyelectrolytes in Water Treatment.” Journal American Water Works Association, 83(10), 88-91.
[16] ASTM (American Society for Testing and Materials).
1995.Standard practice for coagulation2flocculation jar test of
water E1-1994 R(1995), D 2035-80. Pages in Annual book of
ASTM standards, volume 11.02.
[17] Okoli C (2012) “Development of Protein-Functionalized Magnetic Iron Oxide Nanoparticles: Potential Application in Water treatment (Doctoral Thesis).
IJSER © 2013 http://www.ijser.org