International Journal of Scientific & Engineering Research, Volume3, issue 3, March,2012 1
ISSN 2229-5518
Removal of Cr (VI) from Water by Using Activated Carbon prepared from
Crotalaria burhia
Suresh Chandra*, Kailash Daga, Vinod Vaishnav, Madan Lal, Bhanupriya Mordhiya
Environ –Industrial laboratory, Department of Chemistry
JAI NARAIN VYAS UNIVERSITY, JODHPUR (RAJASTHAN) INDIA
E-mail:Chandra_sureshp@yahoo. Com Corresponding Email-vaishnav.vinod2@gmail.com ABSTRACT
The awareness of increasing water pollution implies studies concerning water treatment. Removal of heavy metals from industrial wastewater is of primary importance1. With a rapid increase in global industrial activities, pollution derived from the uncontrolled escape of heavy
metals such as copper, nickel, chromium and zinc has become serious. The awareness of increasing water pollution implies studies concerning water treatment. The presence of toxic heavy metals such as chromium (VI) contaminants in aqueous streams, arising from the discharge of untreated metal containing effluents into water bodies, is one of the most important environmental problems. Adsorption is one of the effective techniques for chromium (VI) removal from wastewater. Industrial effluent containing Cr (VI) plays an important role in polluting water bodies. The major objective of this paper was to investigate the removal of Cr (VI) from synthetic wastewater using poly vinyl alcohol coated activated carbon prepared from
stem of Crotalaria burhia 2. The effect of various parameters such as contact time, adsorbent dosage, initial chromium (VI) concentration and pH has been studied. The chromium (VI)
adsorption followed both the Langmuir3and Freundlich’s equation isotherms4.
Key Words: Chromium (VI), Adsorption isotherms, Crotalaria burhia
Introduction: The awareness of increasing water pollution implies studies concerning water treatment. Removal of heavy metals from industrial wastewater is of primary importance1. The discharge of heavy metals into aquatic ecosystems has become a matter of concern over the last few decades. The presence of
heavy metals in the environment is of major concern because of their toxicity to many life forms. Numerous industrial processes produce aqueous effluents that contain heavy metal contaminants. Since the majority of heavy metals do not degrade into harmless end products, their concentrations must be reduced to acceptable levels prior to
discharge of industrial effluents16. Otherwise, they could pose threats to public
health and/or affect the aesthetic quality of potable water. According to the World
Health Organization (WHO), the metals of most immediate concern are aluminum,
chromium, manganese, iron, cobalt, nickel, copper, zinc, cadmium, mercury and lead17. These toxic materials may be derived from mining operations, refining ores, sludge disposal, fly ash from incinerators, the processing of radioactive materials, metal plating, or the manufacture of electrical
equipment, paints, alloys, batteries, pesticides or preservatives. The commonly used procedures for removing metal ions from effluents include chemical precipitation, lime coagulation, ion exchange, reverse osmosis and solvent extraction These techniques apart from being economically expensive have disadvantages like incomplete metal removal, high reagent and energy requirements, and generation of toxic sludge or other waste products that require disposal. Efficient and environment friendly methods are thus required to be developed to reduce heavy metal content. In this context,
International Journal of Scientific & Engineering Research, Volume3, issue 3, March,2012 2
ISSN 2229-5518
considerable attention has been focused in recent years upon the field of adsorption for the removal of heavy metal ions from aqueous effluents. Toxicity of chromium compounds depends on its oxidation states, i.e. chromium (III) and chromium (VI), which are regulated in different ways. Chromium (VI) was recognized to be much more toxic than chromium (III). Hexavalent chromium, being strong oxidizing agent is very reactive in solution .It is known to be a carcinogen and is generally believed to be a more toxic of the two-oxidation states. However, there is sufficient evidence available to warrant consideration of both
wastes of chromium as being highly toxic7.
Hexavalent chromium is known for its negative health and environmental impact, and its extreme toxicity. Health effects related to hexavalent chromium exposure include diarrhoea, stomach and intestinal bleedings, cramps, and liver and kidney damage, skin ulceration, perforation of the nasal septum and lung cancer. Chromium and its compounds are widely used in electroplating, leather tanning, cement, dyeing, metal processing, wood preservatives, paint and pigments, textile, steel fabrication and canning industries. These industries produce large quantities of

toxic wastewater effluents 11.
Materials and Methods
Adsorbent material
The adsorbent prepared by the naturally
dried stems of the plant Crotalaria burhia obtained locally are used in the studies. The material was cut into small pieces. The stem were treated with concentrated sulphuric acid and kept in oven at 1500C for 24 hours. It was filtered and washed with distilled water repeatedly to remove sulphuric acid (washing tested with two drops of barium
chloride solution) and finally dried. The adsorbent was sieved to 40-60-mesh size and heated at 1500C for 2 hours. This
material was used as adsorbent for removing chromium.
Preparation of stock solution
(Synthetic wastewater)
An aqueous stock solution (1000 mg/l)
of Cr (VI) ions was prepared using
K2Cr2O7 salt. pH of the solution was adjusted using 0.1 N HCl or NaOH. Fresh dilutions were used for each study. Adsorption studies
The adsorption capacity of Crotalaria burhia was determined by contacting various concentrations (40 - 150 mgl-1) of 100 ml Chromium solution in 250 ml conical flasks, with 1.5 gram of Crotalaria burhia. The mixture was shaken in a rotary shaker at 150 rpm followed by filtration using Whatman
filter paper (No. 1). The filtrate containing the residual concentration of Cr was determined spectrophotometerically at 540 nm after complexation with 1, 5 diphenylcarbazide. For the determination of rate of metal adsorption by Crotalaria burhia from 100 ml (at 40, 60, 80,
100,120,150 mgl-1), the supernatant was analyzed for residual Chromium after
the contact period of 15, 30, 45, 60, 75,
90,105,120, 135, and150, and min. The
effect of pH on Cr (Vl) sorption by Crotalaria burhia was determined at pH values of 2, 3, 4, 5, 6 and 8. The effect of different doses of Crotalaria burhia ranging from 3 to 18 g/L at varied Cr (VI) concentrations was determined. Adsorption isotherm studies were carried out with six different initial concentrations ranging from 40 to 150 ppm of Cr (VI) while maintaining the adsorbent dosage at 1.5g/100ml. Langmuir and Freundlich models were applied to the adsorption isotherm and different constants were generated. The Langmuir and Freundlich adsorption parameters and correlation coefficient
International Journal of Scientific & Engineering Research, Volume3, issue 3, March,2012 3
ISSN 2229-5518
were also calculated from the adsorption isotherm data.
Result and Discussion
The data obtained during the
adsorption studies made here to evaluate the effect of contact time, pH of solution, adsorbent doses and initial Cr (Vl) concentration on the removal of hexavalent chromium from the synthetic wastewater have been analyzed and discussed under the following head.
(1.) Effect of contact time on Cr (Vl)
removal:
In adsorption system,
the contact time play a vital role irrespective of the other experimental parameters, affecting the adsorption kinetics. Figure: 1 depicts that there was an appreciable increase in percent removal of chromium up to 105 minutes and thereafter further increase in contact time the increase in removal was very small. Thus the effective contact time is taken as 105 minute.
(2.) Effect of pH on Cr (Vl) removal:
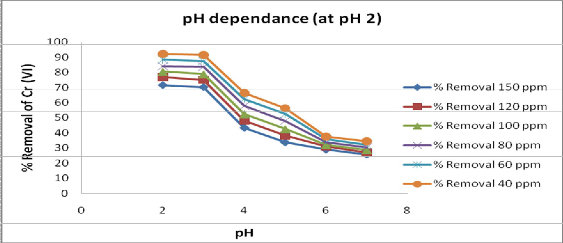
The influence of solution
pH on the extent of adsorption of adsorbate material used is shown in fig.2. The removal of metal ions from solution by adsorption is highly dependent on the pH of the solution.
It was found that 92.2% and 91.4%
removal of Cr (Vl) achieved at pH2 and
3 respectively and there after the percent
removal decreases with increases in pH as 4, 5, 6, and 8.Thus the optimum adsorption pH for Cr (Vl) is 2.
(3.) Effect of dose of adsorbent on Cr
(Vl) removal:
The effect of adsorbent dose on percent
removal of chromium is shown in fig.3. Adsorbent dose was varied (3, 6, 9, 12,
15,18g/L) and performing the adsorption studies at pH2. The present study
indicated that the amount of Cr (Vl)
adsorbed on adsorbent increased with
increase in the adsorbent dose .The higher dose of adsorbent due to an increased surface area would have caused the availability of more number of adsorption sites resulting in higher Cr (Vl) removal.
(4.) Effect of adsorbate concentration: Effect of initial Cr (Vl) concentration over the percent removal of chromium is shown in fig.4 and shows that, as the concentration of chromium in solution increases, the percent removal of chromium decreases .The percent removal with Crotalaria burhia was found 92.2% at a Cr (Vl) concentration of 40ppm. hence the effective adsorbate concentration of chromium is taken as
40mg/l.
(5.) Adsorption capacity:
The amount of metal ions adsorbed per
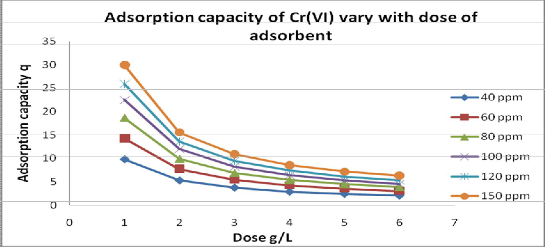
unit mass of adsorbent is called as adsorption capacity .It is increases with metal ion concentration as shown in fig.6 but decreases with increases the concentration of dose of adsorbent as shown in fig.5.
(6.) Adsorption Isotherm:
Langmuir and Freundlich isotherms for
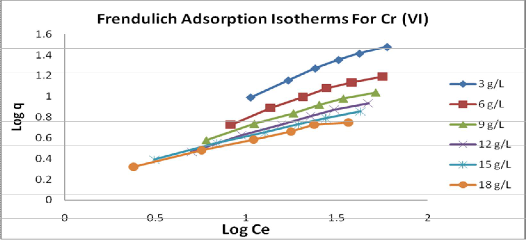
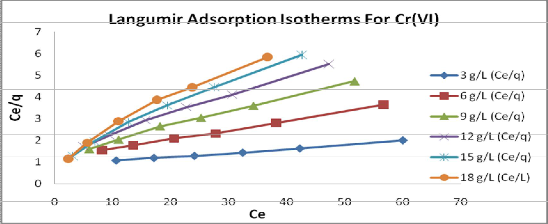
the metal ion are linear, (fig.7and8) showing the applicability of the isotherms. The values of Langmuir and Freundlich constants calculated from the graph are summarized in table 1 and 2. Values of the n are gartering then one, indicating good sorption potential of the sorbent.
Values of dimensionless equilibrium parameter RL show the adsorption to be favorable (0< RL <1). The correlation) coefficients (Cc) for Langmuir and Freundlich isotherms are equal. Therefore, for the present adsorption study, it can be stated that Langmuir and Freundlich adsorption equations found to be better fitted.
Conclusion:
International Journal of Scientific & Engineering Research, Volume3, issue 3, March,2012 4
ISSN 2229-5518
Pollution of the aquatic environment with toxic valuable metal is widespread. Consideration of the modes of purifying these contaminations must be given to strategies that are designed to high thorough put methods while keeping cost at minimum. Adsorption readily provides an efficient alternative to traditional physiochemical means for removing dye. In conclusion, Crotalaria buria could be used as potential adsorbent for the removal of Cr (VI) from aqueous solutions.
Table1. Values of Langmuir and Freundlich isotherm constants for adsorption of Chromium (Vl):
Cc = Correlation Co-efficient.
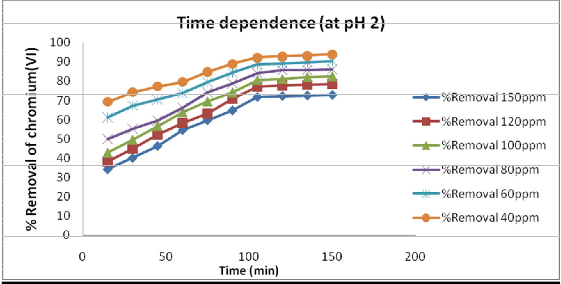
Fig 1: Effect of contact time on adsorption of different concentration of Cr (Vl) pH 2 and
15g/L of adsorbent dose.
International Journal of Scientific & Engineering Research, Volume3, issue 3, March,2012 5
ISSN 2229-5518
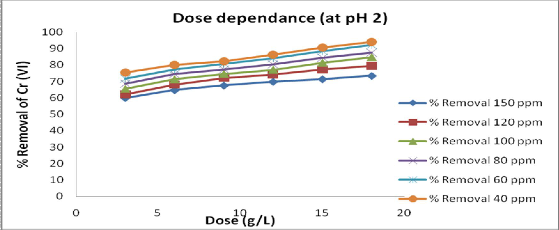
Fig 2: Effect of pH on adsorption of different concentration of Cr (Vl) at constant contact time105 min and 15g/L of adsorbent dose.
Fig 3: Effect of dose of adsorbent at equilibrium contact time 105 min. and 15g/L of adsorbent dose, at effective pH 2.
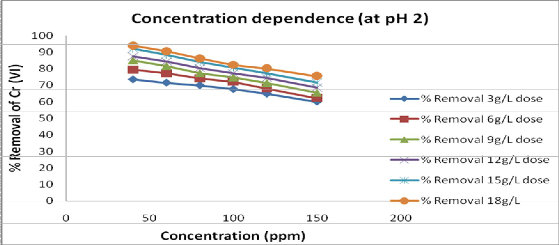
Fig 4: Effect of initial concentration of Cr (Vl) at equilibrium contact time 105 min. and
15g/L of adsorbent dose, at effective pH 2.
International Journal of Scientific & Engineering Research, Volume3, issue 3, March,2012 6
ISSN 2229-5518
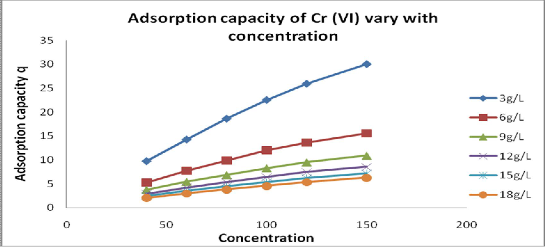
Fig 5: Effect of dose of adsorbent on adsorption capacity at equilibrium contact time 105, and at effective pH 2.
Fig 6: Effect of initial concentration of Cr (Vl) on adsorption capacity at equilibrium contact time
105, and at effective pH 2.
Fig 7: Freundlich Isotherm for effect of different amount of adsorbent used on adsorption of Cr
(Vl) at constant contact time and pH 2.
International Journal of Scientific & Engineering Research, Volume3, issue 3, March,2012 7
ISSN 2229-5518
Fig 8: Langmuir Isotherm for effect of different amount of adsorbent used on adsorption of Cr
(Vl) at constant contact time and pH2.
REFERENCES:
1. APHA, AWWA(1994) Standard Methods for Examination of water and wastewater
19th Edition Washington DC
2. K daga, Adsorption of color from textile wastewater using crotalaria burnia,PMIP,2004-ROURKELA(ORISSA)pp-205-209,2004
3. Langmuir I J 1918, J. Amer. Chem. Society. 40, 1361
4. Freundlich H 1926 Colloid Capillary Chemistry(London; Methuen)
5. Ismadji, S and Bhatia, S. K. Can.J.Chem.Engn. 78: 892-901. 2000.
6. Nassar, M. M. and Magdy, Y. H. Indian Chem. Engr.Section A. 40(1): 27-30. 1999.
7. PARK, S. and JUNG, W.Y. Removal of chromium by activated carbon fibers plated with copper metal. Carbon Sci., 2001, vol. 2, no. 1, p. 15-21.
8. Kapadia, M. J., Farasram, R. P., Desai, D. H. and Bhatt, M. M. Indian J. Environ.
Prot. 21(7): 588-595. 2001.
9. Kato, Y., Hiraoka, S., Tada, Y. and Nomura, T. Can J. Chem. Engn. 76: 441-445.
10. Das, C. P. and Patnaik, L. N. Indian J. Environ. Hlth. 43(1): 21-27. 2001.
11. Raji, C. and Anirudhan, T.S. Chromium (VI) adsorption by sawdust: kinetics and equilibrium. Indian Journal of Chemical Technology, 1997, vol. 4, no. 5, p. 228-236.
12. Manassas and M.Elgeundi, JcHEM.Tech.Biotechnol. 50,275(1991).
13. V.Poots, G.McKay and Healy Wat.Res., 10, 1067(1976).
14. S.Mohan and J.Karthikeyan, Environ.Poll. 97,183(1997).
15. M.Aurelia, D.Mihaela, N.Margareata and L.Agenta, Rev Chim., 40,441(1989).
16. M.A.Baig, Bilal Mehmood and Asif Matin* ISSN: 1579-4377 Electron. J. Environ.
Agric. Food Chem.ISSN 1579-4377 374
International Journal of Scientific & Engineering Research, Volume3, issue 3, March,2012 8
ISSN 2229-5518







