
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014 1197
ISSN 2229-5518
Removal of Congo red from aqueous solution using saw dust as a low cost adsorbent
Muhammad Nawaz, Sharjeel Waqas, Muhammad Usman Tahir, Waqar Ali Khan, Muhammad Jamil
—————————— ——————————
Large quantities of synthetic dyes are extensively used in different industries such as textile, tannery, paint, cosmetic, food and pharmaceutical. These industries produced huge volume of wastewater every year [1]. Dyes containing wastewater without proper treatment cause damage to aquatic environment [2]. They also cause serious health problems to human and animals because they are toxic, mutagenic or carcinogenic [3]. Therefore, the riddance of dyes from wastewater is of significant environmental importance [4]. Dyes are very difficult to biodegrade because of their complex molecular structure [5]. Therefore, conventional treatment processes for the treatment of wastewater are not effectual [1].
————————————————
• Muhammad Nawaz is currently working as lecturer in School of Chemical Engineering, The University of Faisalabad, Faisalabad, Pakistan,
PH-+923457895244 Email: muhammad.nawaz244@yahoo.com
• Sharjeel Waqas is currently working as lecturer in School of Chemical Engineering, The University of Faisalabad, Faisalabad, Pakistan,
PH-+923454556266 Email: sharjeelengineer@gmail.com
• Muhammad Usman Tahir is currently working as assistant professor in School of Chemical Engineering, The University of Faisalabad, Faisalabad, Pakistan,
PH-+923009609252 Email: usmanengr1@gmail.com
• Waqar Ali Khan is currently working as associate professor at Chemical Engineering Department in NFC Institute of Engineering and Fertilizer Research, Faisalabad, Pakistan,
PH-+923334333494 Email: engrwaqarali@gmail.com
• Muhammad Jamil is currently working as a professor in School of Chemical Engineering, The University of Faisalabad, Faisalabad, Pakistan,
PH-+923004382693 Email: professor_jamil@yahoo.com
Physical and chemical technologies such as coagulation and flocculation [6], electrochemical removal [7], photochemical degradation [8], membrane separation [9] and adsorption [10] are used to treat dyes containing wastewater. Among these treatment methods, adsorption process is an efficient treatment process especially when the adsorbent is easily available and inexpensive [11]. Adsorption is a promising alternative wastewater treatment process because of simplicity of operation, easy handling of materials, sludge free process and regeneration capacity [12,13].
Activated carbon (AC) has been used by different researchers with great success in adsorption process for the treatment of dyes containing wastewater [14,15]. AC is expensive and its regeneration also increases the cost of the process [16]. So, there is a need to find alternative low cost adsorbents for the process. Many researchers had used various low cost adsorbents such as rice husk [17], cattail root [18], calcium rich fly ash [19], cashew nut shell [20], orange peel [21], sugarcane bagasse [22] and bentonite [23] for the treatment of dyes containing wasterwater.
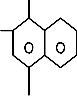
In this study, we use saw dust (SD) as an adsorbent for the adsorption Conge red (CR) from its aqueous solution. CR is an anionic dye and it has been extensively used in rubber, plastic, paper and textile industries [1]. The structure of CR is presented in Fig.1. SD is the by-product of the wood industry and easily available at a very low price.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014 1198
ISSN 2229-5518


adsorbent at different contact times, keeping initial dye
concentration, pH and temperature constant. The results
![]()
![]()
![]()
are shown in Fig.2. It is clear from the Fig.2. that the removal of CR increase with an increase in contact time. When contact time increase from 45 to 90 min, the dye removal increases from 18.2% to 34.5%. There was a slight change observed in the removal of CR after 90 min. So, this
time was considered as an equilibrium time.
Saw dust was collected from a local saw mill at Mongi Bungalow, Toba Tek Singh, Pakistan. For the removal of muddy materials, the saw dust was washed with distilled water and then dried in the sunlight. After that the material was grounded and sieved to desired particle size. Particles between 177-250 microns were selected for experiments. The dried saw dust was activated at 110 °C for 2 hours in a hot air oven and then stored in an air tight container for further use.
The adsorbate Congo red dye was supplied by New Central Chemicals, Lahore, Pakistan. The dye was of analytical grade and 99.8 % pure. It was used as received without further purification. Stock solution of CR was prepared with double distilled water. Experimental solutions were then obtained from the stock solution by dilutions with distilled water.
Batch experiments were carried out by stirring 250 mg of saw dust with 50 ml of aqueous solution of CR of known concentration in 250 ml round bottom flask placed in a temperature controlled rotamentle. The experiments were conducted at different concentrations (betwwen 25-100 mg/l), temperatures (between 25-75 °C, sorbent doses (between 250-1000 mg) and pH (between 5-10) at a constant stirring rate. After the desired contact time, the samples were removed from round bottom flask and centrifuged at 3000 rpm for 2-3 min. after that UV- spectophotometer (set at a wavelength of 500 nm, maximum absorbance) was used to determine the final concentration of CR.
Different experiments were performed for the removal of CR from the aqueous solution using SD as an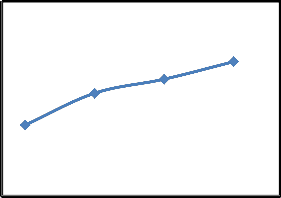
50
40
30
20
10
0
40 50 60 70 80 90 100
(Conditions: T=25°C; pH= 7.1; W /V = 0.25 g/50ml)
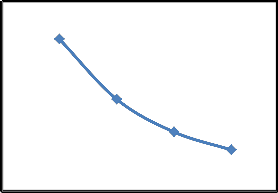
Removal of CR by SD using different initial concentration was studied at 25°C and the results are presented in Fig.3. It is clear from the Fig.3(a), percent removal of CR decreased with the increase in initial concentration but the actual amount of dye adsorbed (mg/g) increased with the increase in initial concentration as shown in Fig.3 (b). When initial concentration of CR increase from
25 to 100 mg/l, the amount of dye adsorbed increases from
2.2 (44.00%) to 5.3 mg/g (26.50%). The removal of CR increased because with the increase in CR concentration, the resistance to up-take of CR from the solution decreased [11]. Rate of adsorption also increases with the increase in initial concentration due to increase in the driving force [11]. Similar results were observed of CR removal on cattail root [18] and calcium rich fly ash [19].
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014 1199
ISSN 2229-5518
50
100
45
80
40
60
35
40
30
25 20
20
0 20 40 60 80 100 120
Initial dye concentration (ppm)
0
0 250 500 750 1000 1250
8
6
4
2
0
0 20 40 60 80 100 120
4
3.5
3
2.5
2
1.5
1
0.5
0
0 200 400 600 800 1000 1200
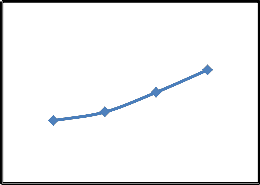
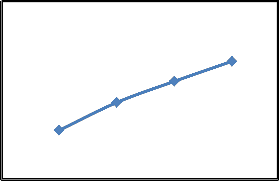
Removal of CR by using different quantities of SD as an adsorbent was studied for 50 mg/l of aqueous solution at 25 °C and pH 7.1. Fig. 4(a) shows that as the quantity of adsorbent increase the removal of CR also increases. When the quantity of SD increases from 0.25 to
1.0 g, the percent dye removal increase from 34.5 % to
62.3 %. CR removal increases because adsorbent surface area and adsorbent sites increases with the increase in the amount of the adsorbent [18]. On the other hand, Fig. 4 (b) shows that amount of dye adsorbed per unit adsorbent decreases with an increase in adsorbent dose. The amount of dye adsorbed (mg/g) decreases from 3.45 to 1.56 as the adsorbent dosage increases from 0.25 to 1.0 g. similar results were obtained by different investigators for the adsorption of CR [14,22].
Percent removal of dye (b). Amount of dye adsorbed (Conditions: T=25°C; pH= 7.1; C o = 50 mg/l, t= 90 min)
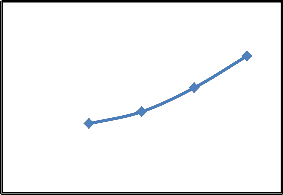
Removal of CR by SD was studied in the range of temperatures between 25 to 75°C for initial concentration
50 mg/l at pH 7.1 and the results presented in Fig. 5. Results shows that amount of dye adsorbed are slightly increased as temperature increases from 25 to 75°C. When temperature increases from 25-75°C, the amount of dye adsorbed increases from 3.45 to 4.86 mg/g as shown in Fig.5. Adsorption capacity may be affected by the diffusion process and chemisorption. If diffusion process is the rate controlling step, then adsorption capacity increases with the increase in temperature due to endothermicity of the diffusion process. With the increase in temperature, mobility of the adsorbate increases and retarding forces acting on the adsorbate decreases [11]. This results in the increase in adsorption capacity. On the other hand, because of sufficient contact time if resistances involved in the diffusion of adsorbate are ignored, chemisorption may involve in the increase in adsorption capacity of SD [11]. Different other researchers has been reported the results similar to the
current study [11,15].
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014 1200
ISSN 2229-5518
6
The Langmuir isotherm is
𝑞𝑚 𝑘𝐿 𝐶𝑒
![]()
5 𝑞𝑒 = 1 + 𝑘 𝐶
𝐿 𝑒
It can be written in linear form as![]()
![]()
![]()
𝐶𝑒 = 𝐶𝑒 + 1
4
Where
𝑞𝑒
𝑞𝑚
𝑞𝑚 𝑏
𝐶𝑒 is the concentration of the dye at equilibrium stage
3 (mg/l), 𝑞𝑒 is the amount of dye adsorbed per unit mass of
adsorbent (mg/g), 𝑞𝑚 and 𝑏 are Langmuir constants. Fig.7.
establish a plot between 𝐶𝑒 verses 𝐶
for the adsorption of![]()
2 𝑞𝑒 𝑒
0 20 40 60 80
CR on SD. The values of 𝑞𝑚 and 𝑏 were calculated from
the slop and intercept of plot between 𝐶𝑒 verses𝐶
and listed
Fig. 5. Effect temperature on the removal of CR by saw dust (Conditions: pH= 7.1; C o = 50 mg/l, W /V = 0.25g/50 50 ml mg, t= 90 min)
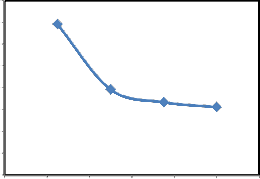
The effect of pH on dye removal by SD was studied at 25°C with 50 mg/l aqueous solution and 0.25 g of SD as an adsorbent. Experiments were performed over a pH range of 5.7-10 and the results obtained are shown in the Fig.6. it can be seen that percent dye removal has inverse relation with pH. 47.5% CR was adsorbed over SD at pH 5.7, while it drops to 25.6% at pH 10. Adsorbent surface charge, speciation of the dye and degree of ionization are affected by the pH of the solution [15]. Electrostatic attraction between CR and SD might be decreased, therefore with the increase in pH of the solution, removal of CR decreased [22]. Similar result has also been observed for activated carbon [15], rice husk [24], kaolin and zeolite [25], however, pH of the solution had negligible effects on CR removal by cattail root and neem leaf powder [18,26].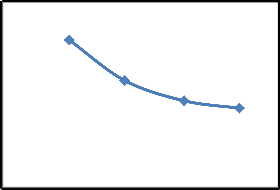
6
5
4
3
2
1
![]()
𝑞𝑒 𝑒
in Table.1. The adsorption capacity calculated in this study
under used conditions was 7.82 mg/g, which is near the value if compared with the calculated adsorption capacities of other researchers [11,14,24].
Congo red on SD at 25°C
Langmuir constant | Freundlich constant | ||||
𝑞𝑚 (mg/g) | b (L/mg) | 2 R | n | k(mg/g) | 2 R |
7.82 | 0.0266 | 0.9882 | 2.9788 | 1.1218 | 0.9621 |
RL , a dimensionless constant, used to determine whether the adsorption is favorable or not and it was calculated by:![]()
1
𝑅𝐿 = 1 + 𝑏𝐶
Where, 𝐶𝑜 is the intial concentration of adsorbate and 𝑏 is
the Langmuir constant. The value of 𝑅𝐿 shows the isotherm
type such that linear (𝑅𝐿 = 1), favourable (0 > 𝑅𝐿 > 1),
unfavourable (𝑅𝐿 > 1) and irreversible (𝑅𝐿 = 0) [27]. Values
of 𝑅𝐿 were calculated and listed in the Table.2. From the
Table.2. it can be seen that value of 𝑅𝐿 was in the range of
0 to 1, which means that Langmuir isotherm was favourable
for the adsorption of CR on SD under the conditions used in this research work.
0
4 5 6 7 8 9 10 11
(Conditions: T=25°C; Co = 50 mg/l, W /V = 0.25g/50 50 ml, t= 90 min)
Langmuir and Freundlich isotherms were used to analyze the data obtained.
The Freundlich isotherm is
𝑞𝑒 = 𝐾𝐹 𝐶𝑒
It can be written in linear form as
1�𝑛
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014 1201
ISSN 2229-5518
Where![]()
1
ln 𝑞𝑒 = 𝑛 ln 𝐶𝑒 + ln 𝐾𝐹
increase of initial dye concentration, adsorbent dosage and temperature while it decreased when we increase the pH of the solution. The experimental data obtained is analyzed
against Langmuir and Freundlich isotherm equations. The
𝐾𝐹 is the adsorption capacity and 1�𝑛
is the adsorption
results shows that the data obtained for the adsorption of
intensity. For favorable adsorption conditions, value of 𝑛
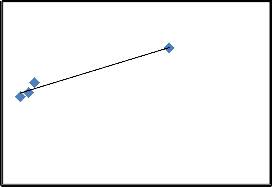
must be in the range of 1-10 [24]. Fig.8. indicates a plot of
ln 𝑞𝑒 verses ln 𝐶𝑒 , gives a straight line with values of slop and intercept shown in the Table.1. The value of 𝑛 calculted
in this study under used conditions was 2.9788 which mean
that Freundlich isotherm was favourable for the adsorption of CR on SD [28].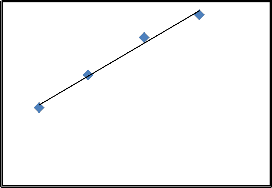
15
12
y = 0.1279x + 4.7983
9 R² = 0.9882
6
3
0
0 20 40 60 80 100
5
4
y = 0.3357x + 3.0704
R² = 0.9621
3
2
1 2 3 4 5
From the present research work, it was found that Saw Dust could be effectively used as an adsorbent for the removal of Congo red from aqueous solution. Effect of initial dye concentration, adsorbent dosage, temperature and pH was studied for the adsorption of CR. Amount of dye adsorbed per unit mass of adsorbent increased with the
CR on SD followed Langmuir and Freundlich isotherms.
The adsorption capacity was found to be 7.82 mg/g. The
value of 𝑅𝐿 calculated from the experimental data are 0.601,
0.429, 0.334 and 0.273 at initial dye concentration of 25,
50, 75 and 100 mg/L respectively which means that adsorption of CR on SW is favourable. Adsorption intensity calculated from Freundlich isotherm shows that SD could be used for the removal of CR.
The authors are greatly thankful to The University of Faisalabad for providing financial and technical support.
2011.
2008.
M. Markazi, F.M. Menger, “The sorption of cationic dyes onto kaolin: Kinetic, isotherm and thermodynamic studies,” Desalination, vol. 266, pp.
274-280, 2011.
M. Arami, “Coagulation/flocculation of dye- containing solutions using polyaluminium chloride and alum,” water science and technology, pp.
1343-1351, 2009.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014 1202
ISSN 2229-5518
BFN from industrial effluents,” Journal of Colloid and Interface Science, vol. 312, pp. 292-296, 2007.
Sikarwar, ”Photochemical degradation of the hazardous dye Safranin-T using TiO2 catalyst,” Journal of Colloid and Interface Science, vol. 309, pp. 464-469, 2007.
of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies,” Journal of Hazardous Materials, vol. 154, pp. 337-346, 2008.
studies on the removal of Congo red from aqueous solution using Eucalyptus wood (Eucalyptus globulus) saw dust,” Journal of the Taiwan Institute of Chemical Engineers, vol. 44, pp. 81-88, 2013.
red dye from aqueous solution using bael shell carbon,” Applied Surface Science, vol. 257, pp.
1628-1633, 2010.
395, 2009.
of congo red using activated carbon and its regeneration,” Journal of Hazardous Materials, vol.
145, pp. 287-295, 2007.
”Modified maghemite nanoparticles as an efficient adsorbent for removing some cationic dyes from aqueous solution,” Desalination, vol. 263, pp. 240-
248, 2010.
Zou, “Use of rice husk for the adsorption of Congo red from aqueous solution in column mode,” Bioresource Technology, vol. 99, pp. 2938-2946,
2008.
of Hazardous Materials, vol. 173, pp. 292-297,
2010.
379, 2004.
Niranjanaa, P. Vijayalakshmi, S. Sivanesan, “Adsorption of dye from aqueous solution by cashew nut shell: Studies on equilibrium isotherm, kinetics and thermodynamics of interactions,” Desalination, vol. 261, pp. 52-60, 2010.
Rani, K. Ranganathan, “Removal of dyes from aqueous solutions by Cellulosic waste orange peel,” Bioresource Technology, vol. 57, pp. 37-43,
1996.
Doherty, “Congo Red adsorption by ball-milled sugarcane bagasse,” Chemical Engineering Journal, vol. 178, pp. 122-128, 2011.
natural bentonite as a cost-effective adsorbent for removal of Congo-red in wastewater,” Journal of Industrial and Engineering Chemistry, 2014.
2009.
”Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials,” Chemical Engineering Journal, vol. 148, pp. 354-
364, 2009.
[27] B.H. Hameed, “Spent tea leaves: A new non- conventional and low-cost adsorbent for removal of basic dye from aqueous solutions,” Journal of Hazardous Materials, vol. 161, pp. 753-759, 2009.
activated sludge hydrolysis and short-chain fatty acids accumulation in the presence of SDBS in semi-continuous flow reactors: Effect of solids retention time and temperature,” Chemical Engineering Journal, vol. 148, pp. 348-353, 2009.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014 1203
ISSN 2229-5518
IJSER © 2014 http://www.ijser.org