International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1641
ISSN 2229-5518
1- Professor ,Central Metallurgical Research and Development Institute (CMRDI), Cairo, Egypt.m naglaaelhussiny@yahoo.com
2- Researcher, Al-Azhar University, Faculty of Engineering, Qena, Egypt.,
3- Assistant Prof. , Al-Azhar University, Faculty of Engineering, Qena, Egypt.,
4- Professor, Al-Azhar University, Faculty of Engineering, Cairo, Egypt.,
Prof.Dr.latif@gmail.com
5- Professor ,Central Metallurgical Research and Development Institute (CMRDI), Cairo, Egypt., shalabimeh@hotmail.com
6- Professor, Al-Azhar University, Faculty of Engineering, Cairo, Egypt, moreda45@yahoo.com
* Corresponding author Shalabi M.E.H. E.Mail :- shalabimeh@hotmail.com
ABSTRACT
This study deals with the grinding of mill scale in a
laboratory ball mill at different milling times, then
briquetting with 2% molasses as binder material. The
effect of grinding of mill scale at different times on
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1642
ISSN 2229-5518
the physicochemical properties and the degree of reduction of mill scale briquettes via hydrogen was investigated. This reaction was found to be controlled by diffusion through reduced layer and experimental
data could be fitted by the equation (1- (1- R)1/3)2 =
kt. The energy of activation was consequently calculated and found to be 28.04 kJ / mole
Key words :- Grinding time , kinetic of reduction vi hydrogen, Energy of activation , mill scale
1. INTRODUCTION
In integrated steelmaking processes, some dusts, sludges, mill scale…etc. commonly called residual materials, are inevitably generated along with the production of steel. Steelmaking by-products very rich in iron (≈
72% Fe), are currently produced in large quantities and represent a potential of almost
5 million tons in the world [1,2]. Dumping of residual materials is not allowed by environmental protection regulations [3]. Generally, these by-products are recycled in metallurgical processes such as the blast furnace or the direct reduction reactors that use coal as reducing agent to produce pre- reduced pellets intended for remelting in electric steel plant. Besides the steelmaking, recycling part of these by-products is
already applied in powder metallurgy where
the economic recovery is more favorable [1,
2].
Mill scale is a steelmaking by-product from the rolling mill in the steel hot rolling process. Mill scale contains both iron in elemental form and three types of iron oxides: Wustite (FeO), Hematite (α-Fe2 O3 ) and Magnetite (Fe3 O4 ). The chemical composition of mill scale varies according to the type of steel produced and the process used. The iron content is normally around 70
%, with traces of non-ferrous metals and alkaline compounds. The reduction of rolling mill scale to sponge iron powder is a new way to take advantage of a cheap by- product of the steelmaking industry, yielding
sponge iron that can be re-used to the
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1643
ISSN 2229-5518
electric furnace as metallic charge for steelmaking to obtain a product with a lower residual content and improved properties [3-
5].
Mechanical activation pretreatment of minerals by intensive milling can improve the efficiency of subsequent processes such as leaching, reduction, chemical synthesis, etc. Consequential benefits of mechanical activation, besides higher efficiencies, may include lower reaction temperatures and enhanced kinetics. As a result, the subsequent processing can be performed in simpler and less expensive reactors with shorter reaction times [6–8]. Several factors, most importantly the formation of new and additional surface area as well as creation of lattice defects, are responsible for the mentioned improvements [6, 9-14]. Different types of milling apparatus such as ball mills, planetary mills, vibratory mills,
pin mills and rolling mills may be used for milling operations [15].
Mechanical treatment in a high energy mill generates a stress field within solids. Stress relaxation can occur via several channels: (1) heat release, (2) development of surface area as a result of brittle fracture of the particles, (3) generation of various sorts of structural defects and (4) stimulation of chemical reaction within solids. All relaxation channels cause changes in the reactivity of the solid substance under treatment, which is why the resulting action is called mechanical activation [16]. The concentration of the mechanically induced defects and their spatial distribution depend upon the condition of the energy transfer in the mill. These can also be influenced by varying the external conditions of stress. The creation of defects enhances the stored energy (enthalpy) in the solids and consequently causes a decrease of activation
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1644
ISSN 2229-5518
barrier for the process and/or subsequent processes [17]. A large number of studies have been devoted to the understanding of the influence of the grinding variables and conditions on the structural changes of mechanically-activated material. Structural changes of inorganic oxides have been shown to be intensified with increasing grinding media density, acceleration and duration of milling in a planetary mill [18]. It has been further reported that the
crystallite size decreased exponentially with
increasing milling time and the strain increased by extending activation time during mechanical activation of ilmenite [19]. A comparative study of the influence of attritor, ball vibratory mills on the reactivity of sulphide minerals was carried out by Balaz et al. [20]. γ-Fe2 O3 samples ground with smaller amplitude in a vibratory mill showed higher reactivity [21]. The aim of this work is to investigate the effect of grinding duration on the quality and the
reduction behaviour of mill scale briquettes.
2. EXPERIMENTAL WORK:
2.1.1. Sieve analysis:
Mill scale used in this experiment was delivered from Egyptian Iron and Steel Company. This mill scale was ground in laboratory ball mill for different times (from
1hour up to 4 hours). This mill scale was
subjected (before and after grinding) to sieve analysis using Tyler standard sieve set with electric shaker. The results are shown in Figure 1 and the mean size of mill scale is
illustrated in Table 1.
Table 1: Mean size of mill scale after grinding for different times
![]()
![]()
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1645
![]()
ISSN 2229-5518
1
0.1
0.01
0.001 Time = 0 hr Time = 1 hr Time = 2 hr
0.0001 Time = 3 hr
Time = 4 hr
1E-05
0.01 0.1 1 10
Figure 1. Sieve analysis of mill scale after grinding in ball mill for different times.
Table 2. Chemical composition of mill scale:
![]()
![]()
3
4
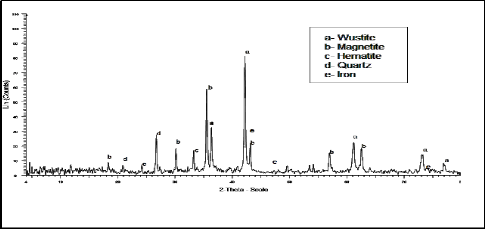
2.1.3. X- ray analysis:
The X- ray analysis of mill scale is illustrated in
Figure 2. It is clear from this figure that it mainly
consists of magnetite, wustite, iron, quartz and
hematite
.
.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1646
ISSN 2229-5518
2.2. Preparation of the briquettes and its physical properties:
After grinding for different times, 10 g of fine mill
scale particles were mixed with 2% molasses then pressed in the mould (12 mm diameter 22 mm height) using MEGA.KSC-10 hydraulic press, under different pressures (from 86 up to 260 MPa). The produced briquettes were subjected to drop damage resistance tests and compressive strength tests. The drop damage resistance indicates how often green briquettes can be dropped from a height of 46 cm
before they show perceptible cracks or crumble. Ten green briquettes were individually dropped onto a steel plate. The number of drops was determined for each briquette. The arithmetical average values of the crumbing behavior of these ten briquettes yield their drop damage resistance, while the average compressive strength is obtained by compressing 10 briquettes between parallel steel plates up to their
breaking[22].
2.3 Reduction Procedure:
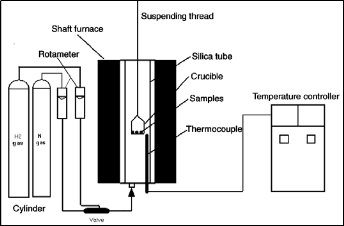
The reduction of mill scale briquette with hydrogen was performed in thermo gravimetric apparatus. This
scheme as shown in Figure 3 is similar to that described elsewhere [23]. The reduction apparatus
consisted of a vertical furnace, electronic balance for
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1647
ISSN 2229-5518
monitoring the weight change of reacting sample and
The percentage of reduction was calculated according
temperature controller. The sample was placed in a
nickel chrome crucible which was suspended under
to the following equation:
Percent of reduction = [(WR0R
–WRtR) ×100/ Oxygen
the electronic balance by Ni-Cr wire. The furnace temperature was raised to the required temperature (600 – 950 °C) and maintained constant within ±5°C.
Then samples were placed in the hot zone of the
mass] --------------------------------------- (1) Where:
WR0R: the initial mass of mill scale sample after
removal of moisture, g;
furnace and nitrogen flown at a rate of 0.5 L/min..
WtR:R
mass of sample after time (t), g;
Air was removed before each experiment and also
after the end of reduction. The weight of each sample
Oxygen mass: indicates the mass of oxygen percent in mill scale in the form of FeO and
was continuously recorded till the end of the run;
then samples were withdrawn from the furnace and put in the desiccators.
FeR2RO
R3,R g.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1648
ISSN 2229-5518
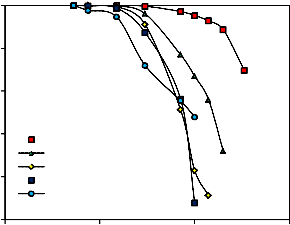
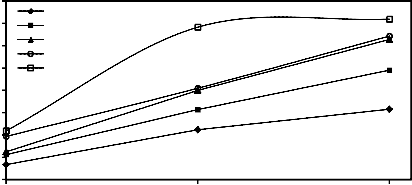
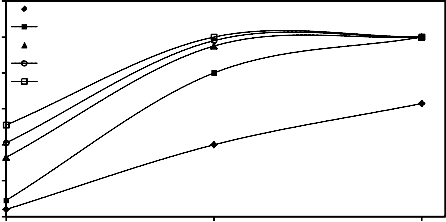
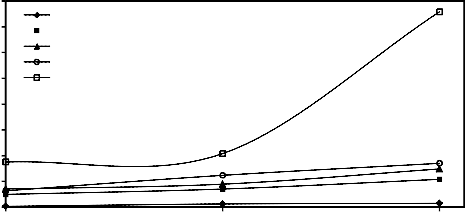
3.1. Effect of pressing load (with constant amount of molasses) on the quality of wet and dry mill scale
briquettes:
Figures 4 to 7 show the relation between the effect of pressing load at constant percentage of molasses (2
%) on the drop damage resistance and compressive strength of both wet and dry briquettes. It is evident that as the pressing pressure load increased both drop damage resistance and compressive strength increased. This is due to the fact that increase of
pressure load increases the compaction of briquette
and subsequently the Vander Waals forces [24, 25]. Also the increase of briquetting pressure leads to progressive crushing of macrospores [26]. These figures also reveal that as the size of mill scale decreases by increasing the grinding time, both drop damage resistance and compressive strength of wet and dry briquette increase.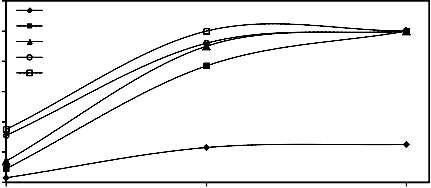
120
100
80
60
Original
1hr. Grin.
2hrs. Grin.
3hrs. Grin.
4 hrs. Grin.
40
20
0
86.7 173.4 260.1
Pressing loads (MPa)
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1649
ISSN 2229-5518
0.35
0.3
0.25
0.2
0.15
0.1
0.05
0
Original
1hr. Grin.
2 hrs. Grin.
3hrs. Grin.
4hrs. Grin.
86.7 173.4 260.1
Pressing loads (Mpa)
120 Original
1hr. Grin.
100
80
2hrs. Grin.
3hrs. Grin.
4 hrs. Grin.
60
40
20
0
86.7 173.4 260.1
Pressing loads (MPa)
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1650
ISSN 2229-5518
8
Original
7 1 hr. Grin.
6 2hrs. Grin.
3hrs. Grin.
5 4hrs. Grin.
4
3
2
1
0
86.7 173.4 260.1
Pressing loads (MPa)
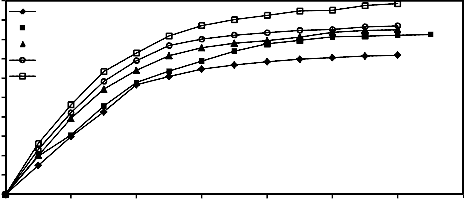
Figure 8 illustrates the effect of increasing milling time on the degree of reduction of mill scale briquettes at 900°C under a pressing load of 260.1 MPa for different times and at 2 L/min. hydrogen flow rate.
From this figure, it is clear that the extent of
the reduction increased as the milling time increased from 1 h. up to 4 h. This can be attributed to the creation of additional surfaces as well as the formation of lattice defects by the action of milling. [6, 9-14]
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1651
ISSN 2229-5518
100
Original
90 1hr.Grin.
80 2hrs.Grin.
70 3hrs.Grin.
60 4hrs.Grin.
50
40
30
20
10
0
0 10 20 30 40 50 60 70
Figure 8. Effect of grinding time and reduction time on the reduction percentage of mill scale briquette (briquette pressing load=260.1
MPa) at 900°C and at 2 L/min. hydrogen flow rate during time progress.
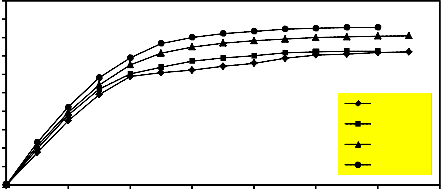
Figure 9 illustrates the relation between the reduction percentage and hydrogen flow rate when the reduction was done at the following constant conditions: Temperature = 900°C, constant weight of the sample, three hours grinding and pressing load =
260.1 MPa). It is clear that as the flow rate of hydrogen increased the reduction percentage increased. The increase of flow rate leads to an
increase of number of hydrogen mole in the bulk
phase, which in turn leads to the raise of hydrogen adsorption and a subsequent increase in the rate of reaction [27, 29]. Also the increase of flow rate increased the gas diffusion across the boundary layer subsequently increasing the extent of reduction [28]. However, from an economical point of view, it is preferable to restrict the flow rate to 1.5 L/min which was adopted in the rest of that investigation.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1652
ISSN 2229-5518
100
90
80
70
60
40
30
20
10
0
0.5L/min.
1L/min.
1.5L/min.
2L/min.
0 10 20 30 40 50 60 70
Time of reduction (minutes)
Figure 9. Effect of hydrogen flow rate on the reduction percentage of mill scale briquette (temperature = 900°C , constant weight of the sample
, 3 hours grinding and pressing load=260.1 Mpa).
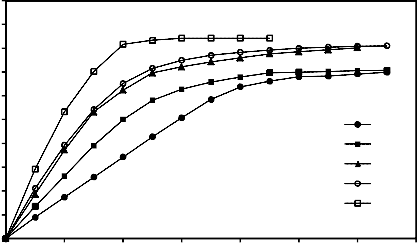
Reduction was carried out at different temperatures ranging from 600 up to 950 °C, where the initial weighs of briquettes were fixed at the same value, the hydrogen flow rate =1.5 liter /min., 3 hours grinding and pressing load=260.1 Mpa. The results are illustrated in Figure 10 for briquettes bound by 2% molasses. It is clear that the increase of temperature
favors both reduction extent and rate. The curves
generally go through three phases: An initial rapid rate, a somewhat slower rate following and a third zone where reduction is slowest. The increase of reduction percentage with rise of temperature is due to the increase of number of reacting moles having excess energy [27, 30]. Also the raise of temperature leads to an increase of the rate of mass transfer of the
diffusion and rate of desorption [28- 31].
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1653
ISSN 2229-5518
100
90
80
70
60
50
40
30
20
10
0
600 °C
700 °C
800 °C
900 °C
950 °C
0 10 20 30 40 50 60 70
Time of reduction (minutes)
Figure 10- Effect of temperature change on the reduction percentage of mill scale briquette at constant condition (temperature = 900°C , 3 hours grinding and pressing load=260.1 MPa).
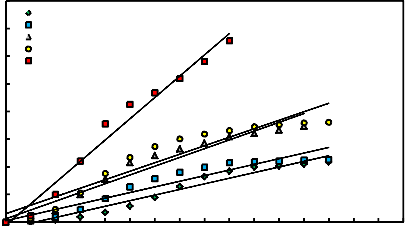
Kinetic studies for the estimation of apparent activation energies were carried out for mill scale briquettes at different temperatures from 600 up to 950oC for different interval times in the range of 0 – 65 minutes.
1- By using diffusion process controls equation (Jander and Anorg Equation) [32]
(1- (1- R) 1/3 )2 = kt ------------------- (2)
Where R is the fractional reduction, t is the time of reduction and k is the rate constant. Figure 11 illustrates the relation between (1- (1- R)1/3)2 = kt and time of reduction for different reduction temperatures. From this figure, it is clear that the relationship can be represented by straight lines.
The Arrhenius equation was used to calculate the activation energies of reduction reaction by using the calculated rate constant
k.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1654
ISSN 2229-5518
![]()
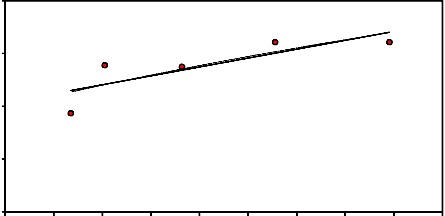
𝑘 = 𝑘₀ exp �− 𝐸 � ------------------- (3)
𝑅𝑇
the natural logarithm of reduction rate
constant and the reciprocal of absolute
![]()
ln𝑘 = ln𝑘0 − 𝐸
𝑅𝑇
------------------- (4)
temperature for mill scale briquettes is
Where k₀ is the pre-exponential coefficientR;R
E is the apparent reduction activation energy
(kJ/mol); R is the universal gas constant (8.314×10-3 kJ/ mol·K); T is the absolute temperature (K). The relationship between
shown in Figure 12, from which it was found that the activation energy was about
28.04 kJ / mole.
0.4
0.35
0.3
0.25
600 °C
700 °C
800 °C
900 °C
950 °C
y = 0.0077x - 0.0058
R² = 0.9773
0.2
0.15
0.1
y = 0.0031x + 0.0148
R² = 0.8991
y = 0.0032x + 0.0058
R² = 0.9453
y = 0.002x + 0.0025
R² = 0.9183
0.05
0
y = 0.002x - 0.012
R² = 0.954
0 5 10 15 20 25 30 35 40 45 50 55 60 65 70 75 80
Time of reduction (minutes)
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1655
ISSN 2229-5518
-7
-6
-5
-4
-3
0.00075 0.0008 0.00085 0.0009 0.00095 0.001 0.00105 0.0011 0.00115 0.0012
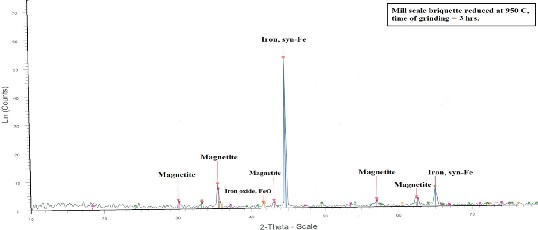
Figure 13 and Figure 14 show the x-ray analyses of two reduced mill scale briquette at 600 and 950 °C
respectively from which it is clear that the main
phases in the reduced briquette are Iron (Syn-Fe), Hematite and Magnetite. Iron oxide (FeO) phase is
present at 950 °C rather than at at 600°C.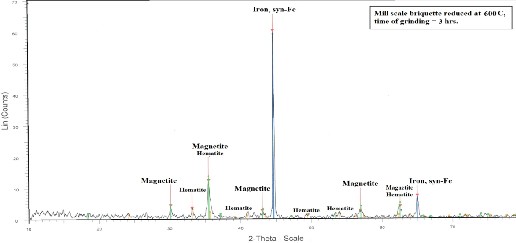
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1656
ISSN 2229-5518
Hydrogen reduction of mill scale ground for different times ranging from 1h to 4h in the form of briquettes (pressing load 260.1 MPa) at temperatures varying from 600 up to 950 ◦C and at 1.5 L/min. hydrogen flow rate for different times indicated that:-
1- The reduction rates increased with increasing temperature of the reduction up to 950 ◦C.
2- Diffusion process controls equation (Jander and Anorg equation) (1- (1- R) 1/3)2 = kt describes the process and the energy of activation was calculated=
28.04 kJ / mole
4.Refrences
(1). Bienvenu, Y., Rodrigues, S. ,”Manufacture of Metal Powders from Pulverulent Waste”, ENSMP, Centre des matériaux, CNRS UMR 7633, France (2007).
(2). O. Benchiheub , S. Mechachti , S. Serrai , M.G. Khalifa., “Elaboration of iron powder from mill scale”, J. Mater. Environ. Sci. 1 (4) 267-276, (2010). (3). L. Camci, S. Aydin, C. Arslan, “Reduction of iron oxides in solid wastes generated by , steelworks”, Turkish J. Eng. Env. Sci., 26 ,(2002) 37-
44.
(4). M.I. Martín, F.A. López, M.E. Rabanal1 and J.M. Torralba,” Production of Sponge Iron Powder by Reduction of a By-product of the Steelmaking Industry”, PM 2010 World Congress – Water Atomized Powders, 1(2010
(5). J.-W. Park, J.-C. Ahn, H. Song, K. Park, H. Shin, J.-S. Ahn, “Reduction characteristics of oily hot rolling mill sludge by direct reduced iron method”, Resour. Conserv. Recy. 34(2) (2002) 129-140.
(6) P. Balaz, L. Takacs, M. Luxova, E. Godocikova,
J. Ficeriova, “Mechanochemical processing of
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1657
ISSN 2229-5518
sulphidic minerals”, Int. J. Miner. Process. 245 (2004) S365–S371.
(7) H. Hu, Q. Chen, Z. Yin, P. Zhang, “Thermal behaviors of mechanically activated pyrites
by thermogravimetry (TG)”,Thermochim. Acta 398 (2003) 233–240.
(8) E. Godocikova, P. Balaz, E. Boldizarova, “Structural and temperature sensitivity of the chloride leaching of copper, lead and zinc from a mechanically activated complex sulphide”, Hydrometallurgy 65 (2002) 83–93.
(9) D. Maurice, J.A. Hawk, “Ferric chloride leaching of a mechanically activated pentlandite–chalcopyrite concentrate”, Hydrometallurgy 52 (1999) 289–312. (10) D. Maurice, J.A. Hawk, “Simultaneous autogenous milling and ferric chloride leaching of chalcopyrite”, Hydrometallurgy 51 (1999) 371–377. (11) J. Ficeriova, P. Balaz, E. Boldizarova,“Combined mechanochemical and thiosulphate leaching of silver from a complex sulphide concentrate”, Int. J. Miner. Process 76 (2005) 260–265.
(12) P. Balaz, E. Boldizarova, M. Achimovicova, R. Kammel, “Leaching and dissolution of a pentlandite concentrate pretreated by mechanical activation”, Hydrometallurgy 57 (2000) 85–96.
(13) N.J. Welham,“Mechanochemical processing of enargite (Cu3 AsS4 )”, Hydrometallurgy 62 (2001)
165–173.
(14) P. Balaz, J. Ficeriova, C. Villachica Leon, “Silver leaching from a mechanochemically pretreated complex sulfide concentrate”, Hydrometallurgy 70 (2003) 113–119.
(15) P. Balaz, “Mechanical activation in hydrometallurgy”, Int. J. Miner. Process 72 (2003)
341–354.
(16) V.V. Boldyrev, K. Tkacova, “Mechanochemistry of solids: past, present, and prospects”, Journal of Materials Synthesis and Processing 8 (3/4) (2000) 121–132.
(17) U. Steinike, K. Tkacova,” Mechanochemistry of solids—real structure and reactivity”, Journal of Materials Synthesis and Processing 8 (3/4) (2000)
197–203.
(18) G.R. Karagedov, N.Z. Lyakhov, “Mechanochemical grinding of inorganic oxides”, Kona 21 (2003) 76–86.
(19) N.J. Welham, D.J. Llewellyn, “Mechanical enhancement of the dissolution of ilmenite”, Minerals Engineering 11 (9) (1998) 827–841.
(20) P. Balaz, H.J. Huhn, K. Tkacova, H. Heegn, “Laugungsverhalten und physico-chemische
Eigenschaften von in unterschiedlichen Mullen
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1658
ISSN 2229-5518
vorbehandeltem chalcopyrite”, Erzmetall 41 (1988)
325–331.
(21) M. Senna, “Criteria of activation of powdery materials by a preliminary mechanical treatment”, Kona 1 (1983) 48–52.
(22) Kurt Mayer,"Pelletization of Iron Ores", Springer-Verlag Berlin Heidelberg,(1980).
(23) El-Hussiny N.A., Shalabi M.E.H., “A self- reduced intermediate product from iron and steel plant waste material using a briquetting process”, Powder Technology, 205, 217223, 2011.
(24) Mohamed F.M., Ahmed Y.M.Z., and Shalabi, M.E.H., , “ Briquetting of Waste Manganese Ore Sinter Fine Using Different Binding Materials”, Environmental issues and waste management in energy and mineral production SWEMP, pp. 567-
573,(2004).
(25) Mangena, S. J. And du Cann, V.M., “ Binderless briquetting of some selected South African prime coking, blend coking and weathered bituminous coals and the effect of coal properties on binderless briquetting”, International Journal of Coal Geology, 71, (2007), pp. 303-312.
(26) Ingles O.G.,“Microstructure in binderless briquetting” , Agglomeration, Edited by William A. Knepper, , Interscience Publishers,(1962). pp.29-53.
(27) Shalabi M.E.H. M.Sc., “kinetic reduction of El- Baharia iron ore and its sinter in static bed by hydrogen”, El- Tabbin Metallurgical Institute for higher studies, Cairo, Egypt, (1973).
(28) Sayed S. A., Khalifa G. M., El-Faramawy E.S.R. and Shalabi M. E. H., “Kinetic Reduction of Low Manganes Iron Ore by Hydrogen,” Egyptian Journal of Chemistry, Vol. 45, No. 1, (2002), pp.47-
66
(29) Sayed S. A., Khalifa M. G., El-Faramawy E.S. R. and Shalabi M. E. H., “Reductions Kinetic of El- Baharia Iron Ore in a Static Bed,” Gospodarka Surowcami Mineranymi, Vol. 17, Special Issue, (2001), pp. 241-245.
(30) Shalabi M.E. H., Mohamed O.A., Abdel-Khalek N.A. and El-Hussiny N.A., “The Influence of Reduced Sponge Iron Addition on the Quality of Produced Iron Ore Sinter,” Proceeding of the XXIMPC, Aachen, (21-26 September 1997), pp. 362-
376.
(31) El-Hussiny N.A., Abdel-Khalek N.A., Morsi M.B., Mohamed O.A., Shalabi M.E.H. and Baeka A. M., “Influence of Water Amount Added on the Sintering Process of Egyptian Iron Ore,” Gornictwo, Vol. 231, (1996), pp. 93-115
(32) Jander W. and Anorg Z., “Kinetic model for
solid – state reactions”, Allgem Chem., (1927).
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1659
ISSN 2229-5518
IJSER © 2015 http://www.ijser.org