International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015
ISSN 2229-5518
123
Production of chitosan from different species of zygomycetes and its antimicrobial activity
Mohamed M. Gharieb; Sabha M. El-Sabbagh, Marwa A. Shalaby and Osama M. Darwesh
Abstract— Zygomycetes fungi cell wall is a source for chitosan production. In this study, chitosan was produced by three fungal strains (Cunninghamella elegans RCMB 012002, Mucor rouxii RCMB 015002 & Rhizopus.sp) and its antimicrobial activity was investigated against deferent pathogenic microorganisms. The chitosan produced by the fungal strains was characterized by FTIR to evaluate the degree of deacetylation, and the DD was 80.30, 81.50 & 80.30 % with Mucor rouxii, Rhizopus sp. & Cunninghamella elegans, respectively. To enhance the value of fungal chitosan production, five culture conditions such as growth medium, carbon and nitrogen source, pH value and temperature were studied. The results showed higher chitosan yield with YPG medium and gave 640, 440 and 240 mg/l for Cunninghamella elegans, Mucor rouxii & Rhizopus sp., respectively. The higher yield of chitosan was obtained when used glucose as carbon source for all fungal strains. The sodium nitrate was the best nitrogen source for Cunninghamella elegans & Mucor rouxii, but urea as the best one for Rhizopus sp. The pH 5 was the best for Cunninghamella elegans & Mucor rouxii, while the pH 4.5 was the best one for Rhizopus sp. and 30ᵒc as temperature for all fungal strains. The produced chitosan was used as antimicrobial agent against pathogenic microbes, Staphylococcus aureus (ATCC- 47077), Escherichia coli (ATCC- 25922), Candida albicans (ATCC- 10231) and Fusarium oxysporum. The results showed variable antimicrobial activity against all tested microorganisms. The minimum inhibition concentration (MIC) of produced fungal chitosan was calculated and the results were ranged between 100 and 1500 pp m with all tested microbes. We produced eco-friendly fungal chitosan and applied it as antimicrobial agent which can be used in various fields.
Key words — Zygomycetes, Chitosan, Antimicrobial activity, FTIR.
—————————— ——————————
1 INTRODUCTION
YGOMYCETES are probably the most ancient group of fungi and traditionally identified as the “pin molds”. They exist as extended mycelia with diverse asexual and
sexual spore structures (Kirk et al., 2008). Unlike most other groups of fungi, the vegetative mycelium of zygomycetes does not contain septa except where the presence of septa is neces- sary for separation of structures such as spores (Kirk et al.,
2008). Zygomycetes fungi are known as fast growers with the capability of formation of sporangiospores (or conidia) and under appropriate circumstances, even zygospores within 1-3 weeks. The quicker growth of these fungi compared to other fungi may be due to lack of septa, leading to faster cytoplas- mic movement or intra-hyphal transportation (Pochanavanich and Suntornsuk 2002). Zygomycetes are the group belonging to the chitin-chitosan category classified by cell wall composi- tion. Chitosan is a distinctive component of the cell wall of zygomycetes fungi and its content can reach up to 3-fold of that of chitin (Aghdam, 2010). Therefore, fungal chitosan pos- sibly boasts an important function in the defence system of zygomycetes by protecting the chitin against hydrolytic attack by chitinases (Synowiecki and Al-Khateeb 2003).
Chitosan, being a cationic polysaccharide, is in the cell wall neutralized with some anionic polymers such as polyphos- phates and polyglucuronic acid (Nwe et al., 2011).
Chitosan is a linear copolymer comprised of randomly repeat- ing glucosamine and N-acetylglucosamine units connected by β→ (1, 4) type linkages. The chemical structure is represented by Figure (1). This polymer can be used as a suitable function- al material, because it possesses desirable properties such as: biocompatibility, biodegradability, non-toxicity and adsorp- tion of fats. Also, it can be used as a flocculating and chelating agent, as a permeability control agent, as a support to immobi-
lize enzymes and as an encapsulating agent, among other ap- plications found in different areas (Aghdam, 2010).
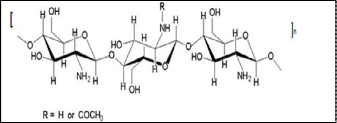
Figure 1. Chemical structure of chitosan.
Chitosan and its derivatives can be variously used as a permeability control agent, an adhesive, a paper-sizing agent, a fining agent, flocculating and chelating agents, an antimi- crobial compound and a chromatographic support. It is also used to deliver drugs to their target (Alves et al. 2008).
The production of chitin/chitosan from microbial sources appears promising because the process can be manipulated to obtain a pure, rather uniform product with specific character- istics. In addition, the fermentative production of fungi on cheap industrial by-products and wastes is an unlimited and, in principle, a very economic source of chitin/chitosan. As well, the feasibility of obtaining β-glucan from the myceliar chitosanglucan complex, and the simultaneous extraction of chitin and chitosan make the microbial process more interest- ing (Pomeroy et al. 2001).
For extraction of chitosan from cell wall, first the cell wall is isolated from the fungal biomass through an alkali treatment (with dilute Na OH solution) at elevated temperature (e.g. 90-
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 6, Issue 3, March-2015
ISSN 2229-5518
124
120%). NaOH solution, in this condition, dissolves proteins, lipids, and alkali-soluble carbohydrates and the cell wall is remaining as alkali insoluble material (AIM). In the next step traditionally, chitosan is separated from AIM by dissolution in an acid solution (e.g. 2– 10% acetic acid at 25–95 °C for 1–24 h). In this step, the other components of cell wall are remaining as alkali insoluble material (AIM). At the end, precipitation of fungal chitosan is accomplished by increasing the pH to 9-10 and chitosan is recovered by centrifugation (Lu et al., 2004).
Chitosan is a cationic, nontoxic, biodegradable, and bio- compatible. It exhibits various potential biological activities, such as antitumor, immune stimulatory, antibacterial and anti- fungal properties (Wu et al., 2005). Extensive research has been conducted to explore its potential applications in various industries. Recently, research has shifted and focused on the possibility of developing chitosan as a natural disinfectant (Pochanavanich and Suntornsuk, 2002). It can also be applied to extend the storage life of fresh fruit and other foods. Much of the interest in the antimicrobial properties of chitosan has focused on the possibility of plant protection (Lu et al., 2004). The exact mechanism of the antimicrobial action of chitosan and its derivatives is still unknown, but several mechanisms have been proposed. Interaction between positively charged chitosan molecules and negatively charged microbial cell membranes leads to the leakage of intracellular constituents (Synowiecki and Al-Khateeb, 2003). Chitosan has a wide varie- ty of applications in waste water treatment, food industry, medical industry, biotechnology, agriculture, cosmetics, pulp and paper industry and membrane technology (Pochanav- anich and Suntornsuk, 2002).
This study aimed to extract of fungal chitosan from three zygomycetes strains namely; Mucor rouxii, Cunninghamella elegans and Rhizopus sp. The culture conditions such as carbon and nitrogen source, growth medium, pH value and tempera- ture were maintained to enhance the fungal chitosan produc- tion. And the antimicrobial activity of produced fungal chi- tosan was evaluated using standard pathogenic microorgan- isms.
2 MATERIALS AND METHODS
2.1 Fungal strains for chitosan production
There are three fungal strains (Zygomycetes); two of them are
Mucor rouxii RCMB 015002 and Cunninghamella elegans RCMB
012002. They obtained from Regional Centre for fungi at
Azhar University. One fungal strain was isolated from moldy
bread, purified and examined it under light microscope to
make sure it belongs to Rhizopus sp. The three fungal strains
were used in chitosan production.
2.3 Cultivation of fungal strains
The fungal strains were activated on yeast pepton glucose agar ( YPG), yeast extract 2 gm, peptone 10gm, glucose 20 gm, agar 15-20 gm/l (Atlas, R.M. 2000) for 4 days at 28 oC±2 until sporulation. The spores were collected, suspended in sterile distilled water and counted to slide 106 spores/ml using a haemocytometer slide. One milliliter from Spore suspension of activated fungal strains was inoculated into Sterilized 250ml Erlenmeyer-flask contained 50ml YPG broth. The flasks were incubated in incubator shaker, under 125 rpm at 28oC ± 2 for three days. After incubation time, mycelia were harvested by filtration (whatman No.4), washed by distilled water, dried and weighted.
2.4 Chitosan extraction
Chitosan extraction was carried out by the method of Syn- owiecki and Al-khateeb (1997). Chitosan was extracted from dried mycelia according to the process involving: deprotein- ization with 2% w/v sodium hydroxide solution (30:1 v/w,
121 oC, 15 min). After filtration, the alkali-insoluble material (AIM) was washed with distilled water until the pH was neu- tral, dried and weighted. One gram of dried AIM was added to 40 ml of 20% acetic acid at 80 oC for 6h. The extract was centrifuged at 4000 xg, 15 min, and collected the supernatant. The pH of the supernatant was adjusted to pH 9.0 with 2N NaOH solution, and centrifuged at (4000 xg, 15 min). The pre- cipitated chitosan was washed with distilled water, 95% etha- nol (1:20 w/v) or acetone (1:20 w/v) and dried at 60 oC to a constant weight.
2.5 Characterization of chitosan using Infrared Spectroscopy
(FTIR)
The chitosan samples were previously dried overnight to 60
°C under reduced pressure and homogenized with 100 mg of potassium bromide (KBr). Discs prepared with potassium
bromide were placed to dry for 24 h at 110 °C under reduced pressure. Infrared ray spectroscopy was performed using by using a Fourier Transform Infrared spectrophotometer (Jasco, Model FTIR-6100, Japan). A blank potassium bromide disc was used as a reference. The maximum absorption bands in- tensity were measured by the baseline method.
2.6 Deacetylation degree (DD)
The degree of acetylation (DA) of chitosan was determined according Reberts method (Roberts.G.A.F.1992, Niamsa, N. & Baimark,Y. 2009) using the relationship between the absorb- ance (A1655/A3450), as shown in the equation:
DD % = (A1655/A3450)* 100/1.33
The deacetylation was calculated using the following equa-
tion:
2.2 Morphological Characterization of Rhizopus isolate
The morphological characterization of Rhizopus isolate was observed through the growth on PDA medium then per- formed microscopic observation of mycelial growth at differ- ent incubation period by light microscope (Olympus cx41).
Deacetylation % =100 - % acetylation.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 6, Issue 3, March-2015
ISSN 2229-5518
125
2.7 Effect of different growth media on chitosan production
Five growth media (Molt yeast pepton glucose) MYPG, (Pota- toes dextrose broth) PDB, sabroud’s media & (yeast peptone glucose) YPG (Atlas, R.M. 2000) were used to obtain the best growth medium for chitosan production by the three fungal strains.
A 250 ml Erlenmeyer flask containing 50ml of each medium was sterilized, the pH of media was adjusted to 5. Each flask inoculated by106 spores /ml and incubated at 28 oC ± 2, and
125 rpm of shaking for 7 days .After incubation time, the my- celia were harvested by filtration (whatman no.4), washed by distilled water and dried at 60˚C to a constant weight. Each dried biomass was subjected to chitosan extraction.
2.8 Effect of Different Carbon Sources on chitosan production
Dox medium was chosen as production media. Several carbon sources were used to study the effect on mycelial chitosan production. To study the effect of various carbon sources on chitosan production, in the production medium, sucrose was substituted with four different carbon sources (glucose, fruc- tose, starch & lactose). All the carbon sources were used at 3% (w/v) concentration. Other parameters were constant (5 pH,
28 oC ±2, 7days). Fungal chitosan yield were determined.
2.9 Effect of Different Nitrogen Sources on chitosan production
To evaluate the effect of different nitrogen sources on fungal chitosan production, sodium nitrate (Na No3) was substituted with three different nitrogen sources (peptone, (NH4)2 SO4 & urea). All the nitrogen sources were used at 0.2% (w/v) con- centration. The rest of the physicochemical parameters were constant (5 pH, 28 oC ±2, 7 days). All procedures were per- formed in triplicate. Fungal chitosan yield were determined.
2.10 Effect of Physical Parameters on chitosan production
In order to determine the effect of initial pH of production media, fermentation runs were carried at initial pH varying from 3.5 to 6. The pH was adjusted using 0.1 N hydrochloric acid and 0.1 N sodium hydroxide.
The effect of temperature on yield of fungal chitosan was stud- ied by incubating the production medium at different temper- ature (20˚C, 25˚C, 30˚C, 35˚C). In all three parameters after incubation, biomass was harvested, filtered through Whatman filter paper no. 4, washed with distilled water and dried at
60˚C to a constant weight. All procedures were performed in triplicate. Fungal chitosan yield were determined.
2.11 Antimicrobial activity assay
Microbial strains
The microorganisms were obtained from the American type culture collection (ATCC; Rockville, MD, USA). The Gram- positive bacterium; Staphylococcus aureus (ATCC- 47077), Gram-negative bacterium; Escherichia coli (ATCC- 25922), yeast; Candida albicans (ATCC- 10231) and fungi; Fusarium ox-
ysporum was obtained from Agriculture Microbiology De- partment, NRC, Egypt. These strains used to evaluate the an- timicrobial activity of fungal chitosan.
Culture medium and inoculums
The stock cultures of microorganisms used in this study were
maintained on nutrient agar slants at 4 oC. Inoculum was pre-
pared by suspending a loop full of bacterial cultures into 10 ml
of nutrient agar broth and was incubated at 37oC for 24 h or
suspending a loopfull of fungal cultures into 10 ml of Potatoes
Dextrose broth (PDB) and was incubated at 37 oC for 24h in case of Candida strain, but at 30 oC for 72h in case of Fusarium
strain.
The counts of bacterial strains were maintained 6*106
CFU/ml. Spore count of fungal & yeast strains was performed
by heamocytometer to 6* 106 spores/ ml. About 60 µl of bacte-
rial suspensions was taken and poured into Petri dishes con- taining 20 ml sterilized nutrient agar medium. Bacterial sus-
pensions were spread to get a uniform lawn culture and the same inoculum of fungal and yeast suspensions was taken and poured into petri dishes containing 20 ml sterilized PDA me- dium.
Antimicrobial activity assay
The agar well diffusion method was applied to detect antimi-
crobial activity (Albayrak, et al., 2010). Wells of 6 mm diame-
ter were dug on the inoculated nutrient agar medium and
PDA medium using sterilized cork borer and 60 µl of chitosan
concentrations dissolved in 0.2 % of acetic acid adjusted at 5.5
pH were added in each well. The wells introduced with 60 μl of 0.2 % acetic acid were used as a negative control. The plates
were allowed to stand at 4oC for 2 h before incubation to pre- vent evaporation of tested samples. The plates were incubated at 37oC overnight and examined for the inhibition zone, in case of bacterial & yeast strain but incubated at 30 oC for
3days in case of fungal strain. The diameter of the inhibition zone was measured in mm.
Minimum inhibitory concentration (MIC)
A bacterial suspension of each tested microorganisms was spread on the nutrient agar plate but yeast & fungal suspen- sions were spread on PDA plate. The wells (6 mm diameter) were dug on the agar plate, and 60 µl of chitosan at different concentrations were delivered into them. The plates were al- lowed to stand at 4oC for 2 h before incubation to prevent evaporation of tested samples. The plates were incubated at
37oC for 24 h under aerobic conditions in case of bacterial &
yeast strains but plates of fungal strain incubated at 30 oC for
72h, then followed by the measurement of the diameter of the inhibition zone expressed in millimeter. MIC was taken from
the concentration of the lowest dosed well visually showing no growth after 24 h.
3 RESULTS
3.1 Morphological Characterization of Rhizopus isolate
The morphological characterization by light microscope based on the taxonomic studies described by Pitt and Hocking (1999) is presented in Figure (2). It was observed that no septum can be seen on the Sporangiophore, the sporangium developed at the end of the sporangiophore was spherical shape. The spo-
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 6, Issue 3, March-2015
ISSN 2229-5518
126
rangiospores also possessed similar spherical Shape. Branch- ing of mycelia at the base of sporangiophore into structure known as rhizoid and stolon can be used to differentiate be- tween Rhizopus sp. and Mucor sp. (Pitt and Hocking 1999). From these results, we can conclude that the fungal isolate belongs to Rhizopus sp.
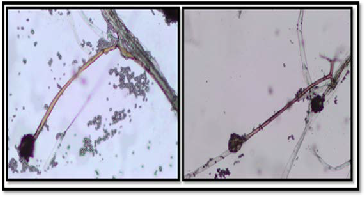
Figure 2. The morphological characterization of Rhizopus sp. by light microscope.
The morphology of Rhizopus grown on PDA medium ob- served by light microscope, are presented in Figure (3) at dif- ferent incubation time. Rhizoids and hyphae at 24 h of growth on PDA medium. Sporangium with sporangiospores at 72h on PDA medium. Sporangium with sporangiospores at 120 h on PDA medium. Rhizoids of Rhizopus at 120 h in PDA medium.
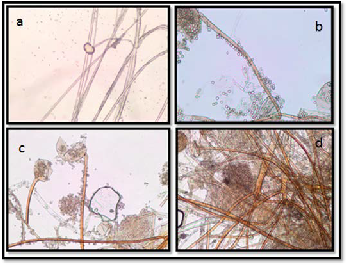
Figure 3. The morphology of Rhizopus sp. on PDA at different incubation time; a, Rhizoids and hyphae at 24 h; b, Sporangi- um with sporangiospores at 72h ; c, Sporangium with sporan- giospores at 120 h; d, Rhizoids of Rhizopus at 120 h.
3.2 Screening of different production media for fungal chitosan production
Screening of production media for fungal chitosan production is presented in Figure (4). Maintaining the fermentation pa- rameters same. In our screening among five production me-
dia, we found that YPG medium gave maximum production of fungal chitosan amount for the three fungal strains, (35 mg/50ml) for Cuninghamella strain, (29 mg/50 ml) for Mucor strain & (13 mg/ 50ml) for Rhizopus strain These results were in agreement with the studies reported by Jaworska & Koniec- zna (2001). YPG was found to contain the highest amount of nitrogen , high nitrogen content increases the synthesis of en- zymes involved in chitin biosynthesis (Andrade et al., 2000), but Dox medium gave minimum production of fungal chi- tosan amount for the three fungal strains (9 mg/50ml) for Cuninghamella strain, (7mg/50ml) for Mucor strain & (6 mg/
50ml) for Rhizopus strain, Dox medium was chosen as produc- tion medium because it is defined medium and it is easy to determine carbon & nitrogen concentration , in addition to Dox medium contains potassium, magnesium & calcium ions that are able to catalyze the activities of chitin deacetylase
,thus increasing the degree of deacetylation of the chitosan
produced (Jaworska and Konieczna, 2001).
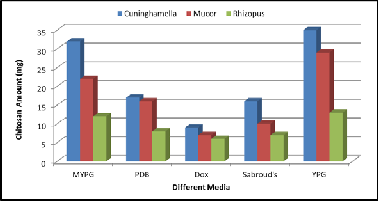
Figure 4. Effect of different growth media on chitosan produc- tion by the three fungal strains.
3.3 Effect of different Carbon Sources on fungal chitosan production
During microbial fermentations, the carbon source not only acts as a major constituent for building of cellular material, but also as an important energy source. All carbon sources with 3
% (w/v) concentration showed different effects on fungal chi- tosan production (Figure 5). The three fungal strains grown on the medium supplemented with fructose, sucrose, glucose, starch & lactose. Among all carbon sources glucose employed showed maximum fungal chitosan production, (20mg/50ml) for Cuninghamella strain, (10mg/50ml) for Mucor strain & (7mg/50ml) for Rhizopus strain. It may be due to the fact that glucose can be easily assimilated in the metabolic pathway for biosynthesis. Glucose was the best carbon source for Mucor rouxii.They found that chitin was accumulated extracellularly in the cell wall by polymerizing activities of chitin synthases with uridine diphosphate Nacetyl glucosamine as a substrate which is biosynthesized from glucose in the cytosol (Dorota et al., 2003).
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 6, Issue 3, March-2015
ISSN 2229-5518
127
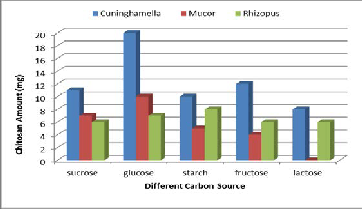
Figure 5. Effect of different carbon sources on chitosan pro- duction by the three fungal strains.
3.4 Effect of different nitrogen sources on chitosan production
The nitrogen source is a critical factor which needs to be opti- mized for fungal chitosan production. Chitosan is a nitrogen containing biopolymer, which is deacetylated form chitin. Fungi require an inorganic or organic nitrogen source as nu- trient to synthesize the chitin and chitosan for their cell wall. Hence the nitrogen source is one of the important factors for the production of chitosan by fungi (Nwe and Stevens 2004). Among all nitrogen sources, sodium nitrate (Na NO3) pro- duced the highest amount for Cuninghamella & Mucor strains (Figure 6), (23 mg/ 50ml) for Cuninghamella strain, (10 mg /
50ml) for Mucor strain, but Urea is the best for Rhizopus strain (13 mg/50 ml). In the absence of nitrogen source, chitosan yield was found to be drastically decreased, hence it can be concluded that that nitrogen source is significant parameter in the fermentative production of fungal chitosan.
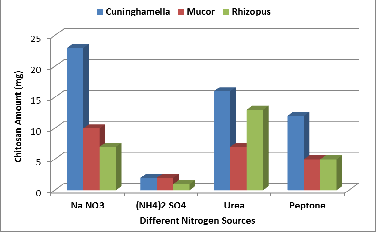
Figure 6. Effect of different nitrogen sources on chitosan pro- duction by the three fungal strains.
3.5 Effect of different initial pH Values on chitosan production
The pH of the medium always influences the physiology of a microorganism by affecting nutrient desirability, enzyme ac- tivity, oxidative-reductive reactions and most importantly cell membrane morphology. Among the various initial pH range studied (3.5 - 6), an initial pH 5 supported the maximum pro- duction of fungal chitosan that for Mucor & Cuninghamella strains (Figure 7). The yield of chitosan was 30 mg/50ml for Cuninghamella strain, (6.8 mg/50ml) for Mucor strain, but pH
4.5 supported the maximum production of fungal chitosan for Rhizopus strain, (10 mg/50ml) for Rhizopus strain. The results were in agreement with the studies reported by Synowiecki and Al-Khateeb (2003). This may be due to the fact that the pH ranging from 4.5 to 5.5, favors the production of enzyme pro- duction chitin deacetylase, which convert chitin to chitosan in fungal cell wall (Arcidiacono and Kaplan 1992).
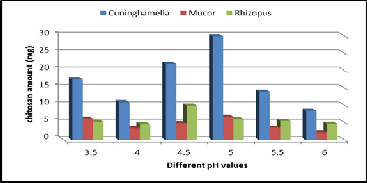
Figure 7. Effect of different pH Values on chitosan production by the three fungal strains.
3.6 Effect of different temperature values on chitosan production
Temperature influences the metabolic activities and microbial growth which affects the production. In order to find out the effect of temperature on fungal chitosan production, fermenta- tion was carried out at different temperatures 20˚C, 25˚C, 30˚C,
35˚C. The yield increased with increase the temperature from
20˚C till 30˚C and further decreased at 35˚C (Figure 8). A max-
imum mycelial chitosan production observed at 30˚C. (17 mg/
50ml) for Cuninghamella strain, (5.7 mg/50 ml) for Mucor
strain, (6.5 mg/ 50ml) for Rhizopus strain.
3.7 Characterization of fungal chitosan using Infrared
Spectroscopy (FTIR)
The IR spectroscopy has been reported as a relatively quick, simple technique and commonly used for qualitative and quantitative evaluations of chitin and chitosan characteristics, mainly their functional groups, the degree of acetyla- tion/deacetylation and impurities (Baxter et al., 1992). It was initially proposed by Moore and Roberts. It has a number of advantages like relatively fast method and does not require dissolution of the chitosan sample in an aqueous solvent. IR spectroscopy is primarily a solid-state method utilizing the concept of baseline for DD calculation. The baseline proposed
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 6, Issue 3, March-2015
ISSN 2229-5518
128
by Baxter et al., (1992) was modified from the method report- ed by Domszy and Roberts (Khan et al., 2002).
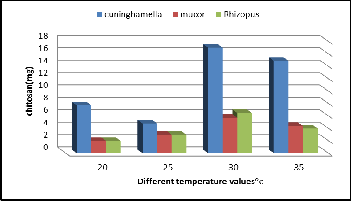
Figure 8. Effect of Different Temperature values on chitosan production by the three fungal strains.
The FTIR spectrum of fungal chitosan from the three fungal strains (Figure 9) Mucor rouxii RCMB 015002, Cunninghamella elegans RCMB 012002 & Rhizopus sp. In this study, the DD val- ue of fungal chitosan obtained from Mucor rouxii was deter- mined to be 80.3%, aiso the same ratio (80.3%) obtained from Cunninghamella elegans but, the DD value of fungal chitosan obtained from Rhizopus sp. was determined to be 81.5% (Table
1).
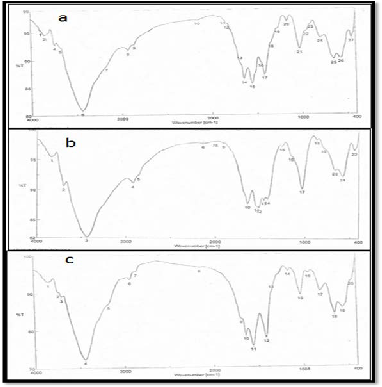
Figure 9. FTIR spectra of (a) fungall chitosan obtained from Mucor rouxii RCMB 015002, (b) fungal chitosan obtained from Cunninghamella elegans RCMB 012002, (c) fungall chitosan obtained from Rhizopus sp.
Table 1. Degree of Deacetylation of chitosan samples pro- duced by the three fungal strains.
Biopolymers | Degree of Deacetylation (DD %) |
Chitosan from Mucor rouxii | 80.30 |
Chitosan from Cuninghamella elegans | 80.30 |
Chitosan from Rhizopus sp. | 81.50 |
3.8 Antimicrobial Activity of chitosan
The exact mechanisms of the antibacterial activities of Chi- tosan (CS) are still unknown. The polycationic structure of quaternized CS is important for antibacterial activity. Electro- static interaction between the polycationic structure and the predominantly anionic components of the microorganisms play a fundamental role in antibacterial activity. The number of ammonium groups linking to the CS backbone is important in electrostatic interaction for the antibacterial activity of CS. It has been reported that quaternized CS with a higher degree of substitution of the quaternary ammonium exhibited a strong interaction with negative charges on the bacterial cell surface (Sajomsang et al., 2009; Kong et al., 2010; Xu et al., 2011). Simi- lar to the antibacterial action, the antifungal activity of CS de- rivatives is believed to occur from the interaction between the cationic chain and the negatively charged residues of macro- molecules exposed on the fungal cell surface, leading to a leakage of intracellular electrolytes and other constituents (Muzzarelli et al., 2001; Martinez et al., 2010). It is believed that CS may affect the morphogenesis of the cell wall, interfering directly with the activity of enzymes responsible for growth of the fungi (EI Ghaouth et al., 1992).
In this study, the antimicrobial activity of fungal chitosan pro-
duced by Cuninghamella, Mucor & Rhizopus strains was
evaluated against tested pathogenic microorganisms; Staphy-
lococcus aurous (ATCC- 47077), Escherichia coli (ATCC-
25922), Candida albicans (ATCC- 10231) and Fusarium ox-
ysporum. The antimicrobial activity was evaluated by agar well diffusion method .The results were measured without
well diameter and represented in Table (2). The fungal chi- tosan was reduced the growth of tested pathogenic microor- ganisms. The effect of produced chitosan was highly value of inhibition zone with Staphylococcus aurous strain and gave
29, 30 and 29 mm when used chitosan extracted from Mucor rouxii, Rhizopus .sp and Cuninghamella elegans, respectively. While, produced chitosan gave low value with E. coli strain (17, 19 and 21 mm) when used chitosan extracted from Mucor rouxii, Rhizopus sp. and Cuninghamella elegans, respectively. According to Zhong et al. (2008) who reported that, the stronger antibacterial activity was apparent against Gram- positive bacteria than Gram-negative bacteria. The effect of fungal chitosan on tested bacterial strains was better than fun-
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 6, Issue 3, March-2015
ISSN 2229-5518
129
gal & yeast strains (Roller and Covill, 1999).
Table (3) presented the Minimum Inhibitory Concentration
(MIC) of prepared fungal chitosan against tested pathogenic
microorganisms. The MIC of fungal chitosan was calculated and the results were ranged between 100 and 1500 ppm with
all tested microbes. Chitosan from Rhizopus sp. was more effective against tested pathogenic microorganisms compared with another sources, this may be due to the highest DD of Rhizopus chitosan (81.5%). This result was agreed with Takahashia et al., (2008); who found that the higher DD with more positive charge was especially successful in inhibiting the growth of S. aureus, suggesting that, the antibacterial ac- tivity of chitosan towards S. aureus enhanced with increasing DD.
Table 2. Antimicrobial activity of fungal chitosan.

an Chitosan
Table 3. Minimum Inhibitory Concentration (MIC) of extract- ed fungal chitosan against pathogenic microorganisms
.
4 REFERENCES
Aghdam, M.G. (2010). Extraction of chitosan from fungal cell wall by Sulfuric acid studying the effect of Deacetylation degree and temperature on recovery chitosan.Msc thesis, School of Engineering, Borås University, Borås, Sweden, 50 p.
Albayrak, S., Aksoy, A., Sagdic, O., Hamzaoglu, E. (2010).
Compositions, antioxidantand antimicrobial activities of
Helichrysum (Asteraceae) species collected fromTurkey.
Food Chem 119, 114-122.
Alves, N.M.; Mano, J.F. (2008). Chitosan derivatives obtained
by chemical modifications for biomedical and environmen-
tal applications. Int. J. Biol. Macromol., 43, 401-414.
Andrade, V.S., Neto, B.B., Souza, W and Campos-Takaki, G.M.
(2000). A factorial design analysis of chitin production by
Cunnighamella elegans. Canadian Journal
Andres, Y.; Giraud, L.; Gerente, C.; Le Cloirec, P.(2007). Anti-
bacterial effects of chitosan powder:Mechanisms of action.
Environ. Technol, 28, 1357-1363.
Atlas,R.M. (2000). Handbook of Microbiological Media. CRC
press, 2nd Ed,New York, ISBN:0-8493-1818-1, 2005p.
Baxter, A., Dillon, M., Taylor, K.D.A. and Roberts, G.A.F.
(1992). Improved Method for I.R. Determination of the De-
gree of N-Acetylation of Chitosan. International Journal of
Biological Macromolecules, 14, 166-169.
Campaniello, D.; Bevilacqua, A.; Sinigaglia, M.; Corbo, M.R.
(2008). Chitosan: Antimicrobial activity and potential ap-
plications for preserving minimally processed strawber-
ries. Food Microbiol, 25, 992-1000.
Dorota, A.B.; Mariusz, O.; Hector, A.L.; Barbara, C.O; Phillips,
W.R. and Charles, A.S. (2003). Chitin synthesis in Sa- charomyces cerevisiae in response to supplementation of
growth medium with glucosamine and cellwall stress. Eu- karyotic cell, 2: 886-900.
EI Ghaouth, A.; Arul, J.; Asselin, A.; Benhamou, N. (1992). An- tifungal activity of chitosan on post-harvest pathogens: In- duction of morphological and cytological alterations in Rhizopus stolonifer. Mycol. Res, 96, 769–779.
Jaworska, M.M. and Konieczna, Z. (2001).The Influence of Supplemental Components in Nutrient Medium on Chi- tosan Formation by the Fungus Absidia orchidis. Applied- Microbiology and Biotechnology, 56: 220-224.
Khan, T.A., Peh, K.K. and Ch’ng, H.S. (2002). Reporting De- gree of Deacetylation Values of Chitosan: The Influence of Analytical Methods. Journal of Pharmacy and Pharmaceu- tical Sciences, 5, 205-212.
Kirk PM, Cannon PF, Minter DW, Stalpers JA. (2008). Diction- ary of the Fungi (10th Ed.). Wallingford, UK: CABI. p.
599.ISBN 978-0-85199-826-8.
Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. (2010). Antimicrobial
properties of chitosan and mode of action: A state of the art
review. Int. J. Food Microbiol, 144, 51–63.
Lu, S.; Song, X.; Cao, D.; Chen, Y. and Yao, K. (2004). Prepara-
tion of Water Soluble chitosan, School of Science, Tianjin
University, People’s Republic of China, J Appl. Polym. Sci.,
91: 3497- 3503.
Martinez, L.R.; Mihu, M.R.; Tar, M.; Cordero, R.J.; Han, G.;
Friedman, A.J.; Friedman, J.M.; Nosanchuk, J.D. (2010).
Demonstration of antibiofilm and antifungal efficacy of
chitosan against candidal biofilms, using an in vivo central
venous catheter model. J. Infect. Dis, 201, 1436–1440. Muzzarelli, R.A.A.; Muzzarelli, C.; Tarsi, R.; Miliani, M.; Gab-
banelli, F.; Cartolari, M. (2001).Fungistatic activity of modi- fied chitosans against Saprolegnia.parasitica. Biomacro- molecules, 2, 165–169.
Niamsa, N. and Baimark, Y.(2009).Preparation and characteri- zation of highly flexible chitosan films for use as food packing. American Journal of food technology, 4(4): 162-
169.
Nwe, N.; Furuike, T. and Tamura, H. (2011). Production,
Properties and Applications of Fungal Cell Wall Polysac-
charides: Chitosan and Glucan. Adv Polym Sci, 244: 187–
208.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 6, Issue 3, March-2015
ISSN 2229-5518
130
Pitt, J.I and Hocking A.D (1999). Fungi and food spoilage (2nd Ed). A Chapman and Hall Food Science Book. Aspen Pub- lisher. Gaiterburg,
Pochanavanich, P. and Suntornsuk, W. (2002). Fungal chitosan production and its characterization. Lett Appl Microbiol.,
35(1):17-21.
Roberts. G.A.F. (1992). Chitin chemistry Macmillan press Itd.,
London, Uk.
Roller, S., Covill, N. (1999). The antifungal properties of chi-
tosan in laboratory media and apple juice. International
Journal of Food Microbiology, 47: 67–77.
Sajomsang, W.; Gonil, P.; Tantayanon, S. ( 2009). Antibacterial
activity of quaternary ammonium chitosan containing
mono or disaccharide moieties: Preparation and character-
ization. Int. J. Biol. Macromol, 44: 419–427.
Synowiecki, J. and Al-Khateeb, N.A. (2003). Production, prop- erties, and some new applications of chitin and its deriva-
tives. Crit Rev Food Sci Nutr., 43(2):145-71.
Synowiecki.J. and Al-khatteb, N.A. (1997). Mycelia of mucr
rouxii as a source of chitin and chitosan. Food-Chem.,
60(4): 605-610.
Takahashia, T., Imaia, M., Suzukia, I., Sawai, J. (2008). Growth inhibitory effect on bacteria of chitosan membranes regu-
lated by the deacetylation degree. Biochemical
Tikhonov, V.E.; Stepnova, E.A.; Babak, V.G.; Yamskov, I.A.;
Guerrero, J.P.; Jansson, H.B.;Llorca, L.V.; Salinas, J.;
Gerasimenko, D.V.; Avdienko, I.D.; Varlamov, V.P. (2006).
Bactericidal and antifungal activities of a low molecular weight chitosan and its N-/2(3)-(dodec-2-enyl) succinoyl/-
derivatives. Carbohydr. Polym, 64, 66-72.
Wu, T.; Zivanovic, S.; Draughon, F.A.; Conway, W.S. and
Sams, C.E. (2005). Physicochemical properties and bioactiv-
ity of fungal chitin and chitosan. J Agric Food Chem.,
53(10): 3888-3894.
Xie, Y.J.; Liu, X.F. and Chen, Q. (2007). Synthesis and charac-
terization of water-soluble chitosan derivate and its anti-
bacterial activity. Carbohydr. Polym, 69, 142–147.
Xu, T.; Xin, M.H.; Li, M.C.; Huang, H.L.; Zhou, S.Q. and Liu,
J.Z. (2011). Synthesis, characterization and antibacterial ac-
tivity of N,O-quaternary ammonium chitosan. Carbohydr. Res, 346: 2445–2450.
Zhong, Z.M., Xing, R.G., Liu, S., Wang, L., Cai, S.B., Li, P.C. (2008). Synthesis of acylthiourea derivatives of chitosan and their antimicrobial activities in vitro. Carbohydrate Research, 343, 566.
————————————————
• Author: Marwa A. Shalaby, Botany Department, Faculty of science, Menoufia Uni- versity, Menoufia, Egypt.
• Co-Authors: Mohamed M. Gharieb and Sabha M. El-Sabbagh, Botany Department, Faculty of science, Menoufia University, Menoufia, Egypt.
• Osama M. Darwesh, Agricultural Microbiology Department, National Research
Center, Cairo, Egypt. Email: darweshosama@yahoo.com.
IJSER © 2015 http://www.ijser.org








