International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 401
ISSN 2229-5518
Preparation of Biodiesel from Higher FFA Containing Castor Oil
Kaniz Ferdous, Anjan Deb, Jannatul Ferdous, Rakib Uddin, Maksudur R. Khan, M.A. Islam
Abstract— Current study reveals the production of biodiesel from non-edible oil such as castor oil (Ricinus Communis L.). Oil was extracted from seed by mechanical press method. Various properties of the raw oil were measured by standard methods. Free Fatty Acid (FFA) content of the oil was found as 13.6% and kinematic viscosity was 253 mm²/s. Base catalyzed transesterification is widely used for biodiesel production than acid catalyzed transesterification due to its faster kinetics. But if the FFA content of the oil is higher than 2wt% then base catalyzed transesterification is not feasible. Hence, a two-step method, acid catalyzed esterification followed by base catalyzed
transesterification was applied for the production of biodiesel from castor oil. After the esterification reaction FFA content of the oil was reduced below 2wt% which makes it suitable for base catalyzed transesterification. The reaction parameters for esterification and transesterification were optimized. 1NMR was studied for both oil and biodiesel. Finally, various properties of produced biodiesel from castor oil, such as specific gravity, viscosity, FFA, cloud point, flash point, saponification value, etc. were measured and compared with standard diesel and biodiesel properties.
Keywords— Biodiesel, Castor oil, Ricinus Communis, Esterification, Transesterification, FFA, Non-edible oil.
—————————— ——————————
RESENTLY the world’s energy needs are met through non-renewable resources such as petrochemicals, natural
gas and coal. Since the demand and cost of petroleum based
but still posed few problems. These problems were namely carbon deposition and lubricating oil contamination. Hence, the most suitable process for reducing the viscosity found was
IJSER
fuel is growing rapidly, and if the present pattern of
consumption continues, these resources will be depleted in
few years. Hence, efforts are being made to explore for
alternative source of energy. An alternative fuel must be
technically feasible, economically competitive,
environmentally acceptable and readily available [1].
Vegetable oils (VOs) have attracted attention as an
alternative for petroleum based fuel [2]. Due to high viscosity
and low volatility, its long term use posed different problems
such as deposition, ring sticking and injector choking in engine [3]. Hence, improvement in the vegetable oil was foreseen to improve the quality of the fuel. To lower the viscosity of vegetable oil, chemical and thermal processes
were tried to make vegetable oil compatible with CI (Compression Ignition) engines. The well-known thermal process, pyrolysis resulted in production of low value materials and sometimes resulted in more production of gasoline instead of diesel. Other processes, i.e. blending and micro-emulsification of vegetable oils reduced the viscosity
————————————————
• Maksudur R. Khan is an associate professor of Faculty of Chemical and
Natural Resources Engineering, Universiti Malaysia Pahang, 26300
Gambang, Kuantan, Pahang, Malaysia, E-mail:mrkhancep@yahoo.com
• M.A. Islam is a professor of Chemical Engineering & Polymer Science
Department of Shahjalal University of Science & Technology, E-mail:
islamsust@yahoo.com
the chemical process, transesterification, where triglycerides
from vegetable oils react with a lower alcohol to produce fatty
acid alkyl esters possessing properties similar to mineral
diesel [4]
Biodiesel is a non-toxic, biodegradable, renewable fuel that
can be produced from a range of organic feedstock including
fresh or waste vegetable oils, animal fats and oilseed plants.
Biodiesel has significantly lower emissions than petroleum-
based diesel when it is burned, whether used in its pure form
or blended with petroleum diesel. It does not contribute to a net rise in the level of carbon dioxide in the atmosphere and leads to minimize the intensity of greenhouse effect [5].
The edible oils such as soybean oil in USA, rapeseed oil in Europe and palm oil in countries with tropical climate such as Malaysia are being used for the production of biodiesel to fuel their compression ignition engines [6]. In Bangladesh, the use of edible oils for engine fuel is not feasible since the potentiality of edible oil sources is below the annual demand [7]; however, there are several non-edible oilseed species such as Castor (Ricinus Communis L.), Nahor (Mesua ferrea.), Pitraj (Aphanamixis Polystachya), Karanja (Pongamia pinnata), Jatropha (Jatropha curcas), Neem (Azadirachta indica), Mahua (Madhuca indica), Simarouba (Simarouba indica) etc., which could be utilized as a potential source for biodiesel production in our country.
Base catalyzed transesterification reaction is widely used for biodiesel production from vegetable oil due to its faster kinetics than that of acid catalyzed process. But if FFA content in the oil is more than 2%, the base catalyzed process is not feasible. The main problem for the non-edible oil sources is its high FFA content which limits the use of single step transesterification reaction. To overcome this problem, two- step procedures were used to prepare biodiesel from different oils, which have high content of free fatty acids [8, 9]. In these
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 402
ISSN 2229-5518
works the first step is the acid catalyzed esterification, which reduces the FFA content of the oil and minimizes the soap formation in the second step (base catalyzed transesterification).
Since the FFA content of raw castor oil was higher, a two- step method was studied for the preparation of biodiesel. Various reaction parameters for esterification and transesterification reaction were optimized. 1H NMR study of oil and biodiesel confirms the elimination of TG in the biodiesel. Finally various properties of the produced biodiesel were measured and compared with biodiesel blend, biodiesel standard and petro-diesel.
Methanol (99-100%), ethanol (99-100%), sodium hydroxide pellets (96%), potassium hydroxide pellets (>84%), phenolphthalein (pH 8.2 - 9.8), starch, acetone (99%), n- Hexane(96%), hydrochloric acid (37%), isopropanol, iodine, sodium iodide, glacial acetic acid, bromine, carbon tetrachloride etc. were purchased from Merck, Germany. All the chemicals used were analytical reagent grade.
Castor seeds were collected from road side of Sylhet region.
methanol and transferred into the reaction medium. The reaction continued until the FFA of oil reduces below 2%. After that the reaction contents were cooled to room temperature and reaction product was washed with hot water until clear water found. The organic phase was collected and dried under vacuum at 100°C for 30 min.
The dried sample (50 ml) was put into a three-necked 500 ml. round bottomed flask, equipped with a reflux condenser, for base catalyzed transesterification. Sodium hydroxide pellets were mixed with required amount of methanol. Molar ratio of methanol to oil was taken excess for the total conversion of TG to biodiesel. The flask was placed on an electric heater with a temperature controller and magnetic stirrer. The transesterification reaction was performed under vigorous stirring at 60°C. After a certain period reaction was stopped by adding stoichiometric amount of HCl. Then contents were cooled to room temperature, and reaction product was washed with hot water until clear water found. The organic phase (biodiesel) was collected and dried under vacuum at 100°C for half an hour. Finally the various properties of the biodiesel were measured.
FFA in the oil and biodiesel samples was analyzed by the method described in AOCS Aa 6-38 [10]. To determine FFA of sample, 4–5 gm of samples were dispersed in isopropanol (75
IJSER
Oil from the seed was extracted by mechanical press method.
Mechanical press is vertical, manual operated, cylindrical (4.3
cm ID) which have a spiral screw that convey the mass from
the hopper to pressure raising area. Slow and continuous
rotation of the mechanical press allows raising sufficient
pressure for the extraction of oil. Here the spiral screw is used
for random mixing, size distribution and passing the mass
from hopper to pressure raising area. Oil drainage nozzles are
located at the face of the expeller. Pressure raising area is
located at the face of the expeller. In this expeller, pressure
rises in slow and continuous rotation. The faster rotation of
the expeller causes the back flow of the solid seed meal and it
results the lowering of pressure. The input of seed grain should be controlled to prevent the loss of the seed because if the amount of raw seed is high, the extra seed meal stays within the screw and it is unable to reach in the pressure
raising area. The back flow of seed grain should be adjusted with the rotation of the spiral screw. After oil extraction it was filtered. The oil was stored at room temperature.
In the current study biodiesel was prepared from castor oil by two step method. A two-step method, acid catalyzed esterification followed by base catalyzed transesterification, of higher FFA containing oil (Castor oil) was adopted for the complete conversion of FFA and TG to fatty acid methyl esters.
The first step (esterification) was carried out at 60°C and atmospheric pressure. Typically, 100 ml oil was taken in a three-necked 500 ml round bottomed flask equipped with a reflux condenser. The flask was placed on an electric heater with a temperature controller and magnetic stirrer. Sulfuric acid was mixed as a catalyst with required amount of
mL) and hexane (15 mL) followed by titration against 0.25 N
NaOH solution. Saponification value (SV) was determined by standard method [11]. Typically, 1 g sample was taken with 25 ml alcoholic KOH solution, heated for 1 hr in a steam bath with occasional shaking and titrated the excess KOH with the
0.5 M hydrochloric acid solution. The iodine value (IV) was determined by the method describe in American Oil Chemist’s
Society. To determine IV, titrating the mixture of tested fuel and chemical reagents against 0.01 N sodium thiosulfate solution until the disappearance of the blue color. IV was calculated by the following Eq. 1:
Iodine value = (B-S) x N x 0.001269/W 1
Where, S and B are the amounts (in unit of mL) of sodium thiosulfate solution required for titration of the tested sample and blank sample respectively. N is the molar concentration (in unit of mol/L) of sodium thiosulfate solution and W is the weight (in unit of gm) of the tested sample.
Physical properties such as moisture content, density and calorific value of the oil were determined by following ASTM D 1744 (Karl Fisher method), ASTM D 1480/81 and ASTM D
240 respectively. Viscosity, flash point, pour point and cloud point were determined by standards ASTM D 445, ASTM D 93 (Pensky–Martens Flashpoint Apparatus, Lazer Scientific Inc., Germany), ASTM D 2500 and ASTM D 97 respectively.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 403
ISSN 2229-5518
Oil was extracted from the seed by mechanical press method. The oil content of Castor oil was found as 48.3% (v/wt). Physical properties of the oil were measured by standard method and presented in Table 1.
at a catalyst (H2 SO4 ) concentration of 2 wt% of oil and a temperature of 60°C for 1 hour.
Sulfuric acid was used as catalyst to enhance the rate of the reaction. The reaction was carried out at different catalyst concentrations and the results are represented in the Fig. 2.
TABLE 1
Properties of Raw Castor Oil
![]()
![]()
FFA Content (wt %) 13.6
Viscosity (mm2/s) 253
Saponification Value (mg KOH/gm Oil) 232.1
Molecular Weight * 876.92![]()
*Molecular weight was determined from the composition of the castor oil. [12]
1.80
1.75
1.70
1.65
1.60
1.55

1 2 3 4 5 6 7
Catalyst Concentration (wt% of Oil)
Biodiesel was prepared from higher FFA containing castor oil by two-step methods. Steps are acid catalyzed esterification
Fig. 2: Effect of catalyst concentration on esterification reaction. [Oil: Methanol = 1:6, Reaction temperature = 60°C, Reaction time = 1 hr.]
IJSER
followed by base catalyzed transesterification.
Acid catalyzed esterification reaction was carried out to
reduce the FFA content of raw oil below 2wt% to make the
transesterification reaction feasible in the second step. Various parameters that affect the esterification reaction are oil to methanol molar ratio, catalyst concentration, reaction temperature and time. All the reaction parameters were
optimized and the results are discussed below.
Since esterification is a reversible reaction higher molar
ratio of alcohol results in a greater ester conversion in a
shorter time. The esterification reaction was studied at
different oil to methanol molar ratio and the results are
represented in Fig. 1.
14
12
10
8
6
4
From Fig. 2 it was observed that with a catalyst concentration of 2 wt% it requires 1 hr for the reduction of FFA below 2%. Reactions were conducted at oil to methanol molar ratio of 1:6 with a temperature of 60°C for 1 hour.
Temperature is an important parameter that affects the esterification reaction. There is an increase in the rate of reaction with the increase in temperature. The reaction was carried out at five different temperatures with 1:6 oil to methanol molar ratio and a catalyst concentration of 2 wt% of oil for 1 hr. The results are represented in the Fig. 3.
2.3
2.2
2.1
2.0
1.9
1.8
1.7
1.6
1.5
1.4
1.3
2
0
0 2 4 6 8 10 12
Oil to Methanol molar ratio
30 40 50 60 70
Temparature (oC)
Fig. 3: Effect of temperature on esterification reaction. [Oil: Methanol = 1:6, Catalyst (H2SO4) concentration = 2 wt% of oil, Reaction time = 1 hr.]
Fig. 1: Effect of oil to methanol molar ratio on esterification reaction. [Catalyst (H2SO4) concentration = 2 wt% of oil, Reaction temperature = 60°C, Reaction time = 1 hr.]
From Fig. 1 it is observed that FFA reduced below 2 wt% at a molar ratio of 1:6 within 1 hour. The reaction was carried out
From fig. 3 it is seen that the maximum reduction in FFA occurs at 60°C. Further increase in temperature shows negative impact since a temperature greater than the boiling point of methanol causes a significant loss of methanol and shows less conversion.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 404
ISSN 2229-5518
Optimum reaction time for esterification reaction was
studied and the results are presented in fig. 4.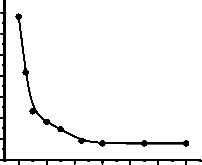
14
12
10
8
6
4
2
0
0 20 40 60 80 100 120
90 90
80 80
70 70
60 60
50 50
40 40
30 30
20 20
10 10
0 0
0.50 0.75 1.00 1.25 1.50 1.75 2.00
Catalyst Concentration (wt%)
Time (min)
Fig. 4: Effect of time on esterification reaction. [Oil: Methanol =
1:6, Catalyst (H2SO4) concentration = 2 wt% of oil, Reaction temperature = 60°C.]
From fig.4 it is seen that the optimum time for esterification reaction is 1 hr.
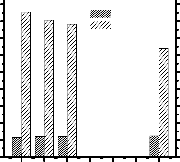
After esterification reaction FFA reduces below 2wt% within 1 hr but viscosity reduces from 253 mm2/s to 81.3 mm2/s. To reduce the viscosity of the product of esterification
Fig. 6: Effect of catalyst concentration on transesterification reaction. [Oil: Methanol = 1:6, Reaction temperature = 60°C, Reaction time = 30 min.]
From fig.6 it is seen that higher concentration of catalyst decreases the reaction yield and increases the viscosity of the biodiesel. This is because the more concentration of catalyst the more tendency of soap formation which converts to FFA during catalyst neutralization. Higher percentage of FFA enhances the final viscosity of the biodiesel. The minimum concentration of catalyst that requires for the completion of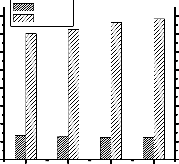
IJSER
was subjected to base catalyzed transesterification. Various
parameters that affect the transesterification reaction are again
the oil to methanol molar ratio, catalyst concentration, reaction
temperature and time. All the results are represented in the
following figures.
Transesterification reaction was carried out at 4 different oil
to methanol molar ratio and results are presented in fig.5.
reaction was 0.5 wt% of oil which was the optimum
concentration of catalyst for transesterification.
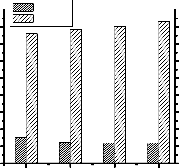
Transesterification reaction was carried out at four different
temperatures to show the effect of temperature on it and the
results are presented in fig.7.
Viscosity
Viscosity
% Yield
80 80
70 70
60 60
50 50
40 40
30 30
20 20
10 10
0 0
80 % Yield 80
70 70
60 60
50 50
40 40
30 30
20 20
10 10
0 0
30 40 50 60
Temperature (oC)
3 6 9 12
Oil to Methanol molar ratio
Fig. 5: Effect of oil to methanol molar ratio on transesterification reaction. [Catalyst (NaOH) concentration = 1 wt% of oil, Reaction temperature = 60°C, Reaction time = 30 min.]
From fig.5 it is seen that viscosity reduces from 81.3 mm2/s to 12 mm2/s at 1:6 oil to methanol molar ratio. Further increase in methanol molar ratio does not reduce the viscosity significantly but increases the reaction yield.
NaOH was used as a catalyst to enhance the rate of transesterification reaction. Reaction was carried out at four different concentration of catalyst and the results are
presented in fig.6.
Fig. 7: Effect of temperature on transesterification reaction. [Oil: Methanol = 1:6, Catalyst (NaOH) concentration = 1 wt% of oil, Reaction time = 30 min.]
From fig.7 it is seen that the optimum temperature for transesterification reaction is 60°C.
Effect of reaction time is represented in fig.8, which shows that, the optimum time for transesterification reaction was 30 min.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 405
ISSN 2229-5518

90
80
70
60
50
40
30
20
10
0
0 10 20 30 40 50 60
Time (min)
Fig. 8: Effect of time on transesterification reaction. [Oil: Methanol = 1:6, Catalyst (NaOH) concentration = 1 wt% of oil, Reaction temperature = 60°C.]
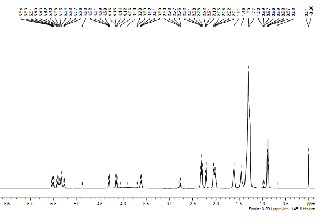
Fig. 10 (a): 1NMR of castor oil
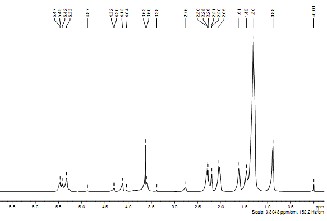
1H NMR spectroscopy of biodiesel and castor oil (CO) was done to investigate the presence of triglyceride backbone in the sample. Fig. 10a, 10b and 10c depicts the 1H NMR spectrum of the CO, after esterification of oil and biodiesel from CO in the region of 0–11.5 ppm. Glyceride protons of triglycerides are assigned in the range of 4–4.5 ppm. The protons of the methyl ester moiety and the a-carbonyl methylene groups are assigned at approximately 3.7 ppm and
2.3 ppm respectively [13]. It can be seen that the glyceride backbone of triglyceride is still present after the esterification but totally absent after transesterification reaction.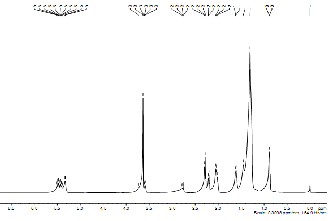
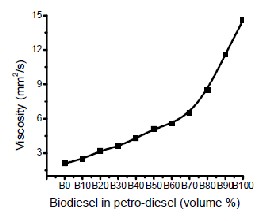
Fig. 10 (b): 1NMR of castor oil after esterification.
Fig. 9: Blending curve of biodiesel with petrodiesel.
The important properties of castor oil methyl esters are compared with biodiesel and petro-diesel standards [14, 15]. The viscosity and specific gravity of the produced biodiesel was higher than biodiesel standard. This biodiesel was blended with diesel with different volumetric proportion. The results are presented in Fig. 9. From the figure it can be seen that the mixture of 70% biodiesel and 30% diesel by volume named B70 match with the physical properties of biodiesel standard. The properties of B100 & B70 are given in Table 3.
Fig. 10 (c): 1NMR of castor oil biodiesel
Biodiesel was prepared from non-edible higher FFA containing castor oil by two-step method. Various reaction parameters for both steps (esterification and trans- esterification) were optimized. Optimum parameters are given in Table 2. The viscosity of the oil was reduced from 253 mm2/s to 11.6 mm2/s and the FFA content was reduced from
13.6 wt% to 1.02 wt%. Viscosity was slightly higher than the
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 406
ISSN 2229-5518
biodiesel standard. The produced biodiesel from castor oil can be used in diesel engine by blending with petro-diesel. B70 was a mixture of 70% by volume of produced biodiesel and 30% by volume of petro-diesel. The viscosity of B70 is similar with biodiesel standard. NMR study confirms the removal of TG backbone in the biodiesel. This product can be successfully used as diesel in diesel engine.
TABLE 2
Optimum Reaction Parameters
[1] A. Srivastava, R. Prasad, “Triglycerides-based diesel fuels”, Renew.
Sust. Energ. Rev. Vol. 4 (2), pp. 111–133, 2000.
[2] H. Noureddini, X. Gao, R.S. Philkana, ``Immobilized Pseudornona cepacia lipase for biodiesel fuel production from soybean oil’’, Bioresource Technology, Vol. 96, pp. 769-777, 2005.
[3] PR Muniyappa, SC Brammer, H. Noureddini, “Improved conversion of plant oils and animal fats into biodiesel and co-product”, Bioresource Technology, Vol. 56, pp. 19–24, 1996.
[4] Ma Fangrui, M. A. Hanna, “Biodiesel production: a review”,
![]()
![]()
Oil : Methanol 1:6 1:6
Catalyst Conc. 2 wt% 0.5 wt%
Temperature 60°C 60°C
Time 60 min 30 min![]()
TABLE 3
![]()
Properties of Biodiesel
Bioresource Technology, Vol. 70, pp. 1–15, 1999.
[5] G. Vicente, M. Martinez, J. Aracil, ``Integrated biodiesel production: a comparison of different homogeneous catalyst systems’’, Bioresource Technology, Vol. 92, pp. 297–305, 2004.
[6] G. Knothe, Curr. Perspect. Biodiesel Inform. 13, pp. 900–903, 2002.
[7] A.K. Kaul, M.L. Das, Promoting the conservation and use of underutilized and neglected crops, Ministry of Agriculture, Dhaka, 2011.
[8] Y. Wang, S. Ou, P. Liu, Z. Zhang, “Preparation of biodiesel fromwaste cooking oil via two-step catalyzed processes”, Energy
Conversion and Management, Vol. 48, pp. 184–188, 2007.
![]()
[9] L.C. Meher, M. Naik, S.N. Naik, L.M. Das, “Production of biodiesel from high free fatty acid Karanja (Pongamia pinnata) oil”, Biomass and Bioenergy, Vol. 32, pp. 354–357, 2008.
Color Pale
Yellow
Pale - -
Yellow
[10] Official Methods and Recommended Practices of the American Oil
Chemists Society, 5th ed, AOCS Press, Champaign. pp. 6-38, 1998.
IJSER
Specific
Gravity
@ 25°C
0.9 0.88 0.885 8.835
[11] G.H. Jeffery, J. Bassett, J. Mendham, and R.C. Denney, Vogel’s textbook of quantitative chemical analysis (5th edition, UK: Longman Scientific and Technical), pp. 308, 1991.
Viscosity
@ 25°C
(mm²/s)
11.6 6.3 1.9 - 6
@ 40°C
1.3 - 4.1
@ 40°C
[12] http://www.groshea.com/pdf/white%20papers/beanbookchemistry. [13] G. Knothe, “Monitoring a progressing transesterification reaction by
fiber-optic near infrared spectroscopy with correlation to 1H nuclear
%FFA
(wt%)
Saponifica- tion value (mg KOH/ gm Oil) Iodiene value Cetane Index Calorific Value (Mj/kg) Flash Point (°C)
Cloud
Point (°C)
Pour Point![]()
(°C)
1.1 1.8 Trace -
212 210.3 - -
71.8 70.2 - -
55.9 56.5 - -
36.25 37.5 39.76 42.25
183 168 100 - 170 60 - 80
3 3 -3 to 12 -15 to 5
2 2 -15 to 10 -35 to -15
magnetic resonance spectroscopy”, J. Am. Oil Chem. Soc., Vol. 77(5), pp. 489–493, 2000.
[14] DYC Leung, Wu Xuan, MKH. Leung, “A review on biodiesel production using catalyzed transesterification”, Applied Energy, Vol.
87, pp. 1083–1095, 2010.
[15] RM. Joshi, MJ. Pegg, “Flow properties of biodiesel fuel blends at low temperatures”, Fuel, Vol. 86, pp. 143–151, 2007.
IJSER © 2013 http://www.ijser.org