
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015
ISSN 2229-5518
37
Preparation and characterization of some transition
metal complexes of 4-amino-N-(5-sulfanyl-1,3,4- thiadiazol-2-yl)benzenesulfonamide
Bassam Abdulhussein Hasan Alsafee
ABSTRACT —1, 3, 4-thiadiazole – 4–aminobenzenesulfonamide compounds are important because of its versatile biological actions such as antibiotics, antimicrobial, anticonvulsant, anti-diuretics, anti-inflammatory, anti-HIV agent, urinary-tract infection, or antiepileptic drugs. In the present study The solid complexes of Co(III), Fe(III), Cr(III), Cu(II), Ni(II) and with Synthes of 4-amino-N-(5-sulfanyl-1,3,4- thiadiazol-2-yl)benzenesulfonamide have been synthesized and characterized by using the spectroscopic IR,1HNMR, Mass as well as by elemental analyses C,H,N and Molar conductance they were studied . It may be concluded for all the complexes the ligand acts as a bidentate and coordinated through sulfonamide oxygen and thiadiazole nitrogen atoms. This view is further supported by the appearance of a band corresponding to the metal–nitrogen and the metal–Oxygen stretching vibration at 583–597 cm–1 and 476-482 cm–1 in the complexes. The physicochemical data suggest the octahedral geometry for all complexes except for Cu and Ni complexes which where tetrahedral respectively.
—————————— ——————————
Sulfonamides have been used as therapeutic agents for over fifty years. They were first used as antibacterial/antibiotic agents, but their applications have been extended to treat other diseases since. In 1935, Sulfonilamide was identified by Domagk et al. as the active metabolite of the red azo dye known as Prontosil as shown in scheme (1) . Prontosil does not possess any activity in vitro; however it metabolizes in vivo to give the active agent sulfanilamide ; where it can interfere with the process of bacterial DNA synthesis and act as potent antibacterial agent. [1]
![]()
O
O
The term sulphonamides are employed as generic name for the derivatives of para amino benzene sulphonamide (sulphanilamide).the sulphonamide drugs were the first effective chemotherapeutic agents to be employed systemically for the prevention and treatment of bacterial infection in humans . the sulphonamides are bacteriostatic antibiotics with wide spectrum action against most gram- positive bacteria and many gram-negative organisms . Sulphonamides are total synthetic substance that are produced by relatively simple chemical synthesis .The advent of peniclline has diminished the usefulness of sulphonamides. But the research in recent years have
shown that the use of penicillin Cause allergic reactions for
H2N
NH2
H2N
![]()
N N S
![]()
![]()
O
O
S
NH2
NH2
some people, which led to the return of interest in sulphonamides compounds .
Antimicrobial compounds contain sulphonamide (SO2NH2) group .this group (SO2NH2) is also present in other compounds, such as antidiabetic agent (e.g Tolubutamide )
,diuretics (e.g chlorthiazide and its congeners,furosemide
and acetazolamide) and anticonvulsant such as
sulthiame.The sulphonamide exists as white powder ,mildly acidic in character, and thy form water –souluble salts with bases .the pH of sodium salts with some exception,for
Scheme (1)
![]()
Bassam Abdulhussein Hasan Alsafee College of Pharmacy, Thi-qar University. E-mail : bassam_org@yahoo.com
example, sodium sulphacetamide ,is very high when given
intramuscular (IM) ,the marked alkalinity causes damage to the tissues.Microorganisms that may be susceptible in vitro to sulphonamides include
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015
ISSN 2229-5518
38
streptococcus pyongens ,streptococcus pneumonia,haemophilus
influenza,H.ducreyi,nocardia,Actinomycesc,alymmatobacteriu
m granulomatis and Chlamydia trachomatis.The minimal
inhibitory concentration ranges from 0.1 mg/ml for C.trachomatis to 4-64 mg/ml . For E.coli . sulphonamides are selective drugs used to treat urinary tract infections, bacterial respiratory infections ,and gastrointestinal (GI) infection. [2] Aziz-ur-Rehman, A. Siddiqa, M. A. Abbasi, S. Rasool, S. Z. Siddiqui, S. Gul, M. Ashraf and R. Nasar has shown in their literature Sulfonamides find their application in biological systems such as, anticancer, anticonvulsant, antidiuretics, anti-inflammatory, carbonic anhydrase inhibitors and HIV protease inhibitors . Because of bacteriostatic activity, these are utilized for the ailment of urinary-tract infection .[3]
On the other hand :- 1,3,4- Thiadiazole is a sulfur-containing
aromatic heterocyclic with nitrogen atoms at the 3-and 4-
positions and is numbered as shown in its structure 1.1,3,4- Thiadiazole exists in two partially reduced (dihydro-) forms,2 and 3 and named as 1,3,4-Thiadiazolines depending on the position of the double bond. the completely reduced (tetrahydro-)1,3,4-Thiadiazole is known as 1,3,4- Thiadiazolidine 4.1,3,4-Thiadiazole exhibit varying biological activity and are ,therefore find their uses in the fields of pharmaceuticals (acerazolamide as diuretic and 2- amino-1,3,4-Thiadiazole antitumor agent in dogs) and agrochemicals (methidathion as an insecticide and as herbicidic).[4]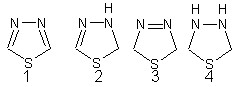
1,3,4-thiadiazole associated with various biological activities such as antimicrobial ,antituberculosis, antiinflammatory, anticonvulsants, antihypertensive ,antioxidant, anticancer and antifungal activity.[5] due to the presence of a toxophoric (-N=C-S-) group . The importance of the sulfur atom in drugs as sulfide or disulfide linkages provides great stability for the three-dimensional structure of the molecule . Besides, the presence of sulfur can have a great contributions to the antimicrobial activities . Disulfide derivatives of 1,3,4-thiadiazole have shown antimicrobial activities against Gram (+) bacteria and fungi [6].
The synthesis of metal sulfanilamide thiadiazole complex has received much attention due to the fact that sulfanilamides were the first effective chemotherapeutic agents to be employed for the prevention and cure of bacterial infections in humans . Furthermore, sulfadrugs and their metal complexes, possess many applications as diuretic,antiepileptic drugs,
among others .The sulfanilamides exert their antibacterial
action by the competitive inhibition of the enzyme dihydropterase synthetase towards the substrate p- aminobenzoate. Several authors have reported the antimicrobial activity of sulfanilamides and their metal complexes.Studies on their metal chelates could have much physiological and pharmacological relevance because the metal chelates of sulfadrugs have been found to be more bacteriostatic than the drugs themselves.[7]
Thus, in this paper we synthesized 4-amino-N-(5-sulfanyl-1,
3,4-thiadiazol-2-yl)benzenesulfonamide some transition
metal complexes . Heavy metals in traces are essential for all forms of life. They are taken up by the living cells as cations. Most of heavy metals play a vital role as co-factors for many important enzymatic reactions in human body. However, coordination metal complexes are gaining increasing importance in the design of respiratory, long acting drugs. Metal ions are therefore known to accelerate drug actions. The efficacies of some therapeutic agents are known to increase upon coordination . due to their coordinating proprieties resulting complexes may exhibit a wide range of biological activities antitumor, antiviral and special biological activities (antimicrobial, anti-inflammatory, antibiotic ,antifungal, antibacterial).[8]
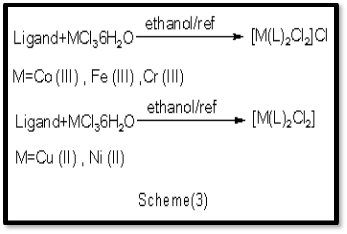
1.Preparation of some transition metal complexes of 4- amino-N-(5-sulfanyl-1,3,4-thiadiazol
-2-yl)benzenesulfonamide as Ligand ,[Co(L)2Cl2]Cl
,[Fe(L)2Cl2]Cl, [Cr(L)2Cl2]Cl, [Cu(L)Cl2], [Ni(L)Cl2] .
2.Identification of synthesized Ligand and Complexes by
Physiochemical data, IR, 1HNMR Spectra, spectroscopic as well as by elemental analyses C, H, N and Molar conductance.
3.Prepared complexes have greater activity and good
models of biological activity systems comparable to Ligand.
1) CS2 2) Thiosemicarbazide 3) absolute ethanol 4) KOH 5)
6) hydrochloric acid 7) Na2CO3 8) pyridine 9) 4-amino benzenesulphonyl chloride.
Elemental C, H and N analysis were carried out on a Thermofinigan flash analyzer, the FTIR spectra in the range (4000-200) cm-1 were recorded as CsI discs using a Shimadzu FTIR spectrophotometer, molar conductance
o
measurements were made in anhydrous DMSO at 25 C
using Inolabcond 720,.The 1H nuclear magnetic resonance
spectra were recorded on a Mercury-300BB NMR 300
spectrometer, DOSO-d6 used as solvent. Melting points
were determined in open capillary tubes using an electro thermal melting point /SMP3I apparatus. Mass spectra were recorded in the range (0-800) m/e on a 5973 network mass selective detector .
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015
ISSN 2229-5518
39
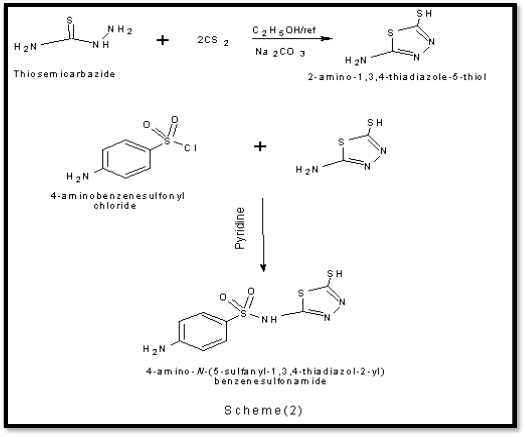
1) Synthesis of 2-amino -1, 3, 4-thiadiazole-5-thiol :- Thiosemicarbazide (,0.043 mole) was suspended in absolute ethanol (30ml) in round bottom flask (250ml), anhydrous sodium carbonate ( 0.021mole) and CS2 ( 0.125mole) were then added respectively with continues stirring. The reactant mixture was refluxed for 5hours; the reaction mixture was then allowed to cool at room temperature and filtered. The filtrate was evaporated under vacuum then cold distilled water (90 ml) was added, acidification with concentrated HCl drop by drop was carried out, white – yellowish precipitate was formed, the white-yellowish precipitate was collected by filtration, and washed with distilled water, re-crystallized by using hot distilled water. The , percentage of yield ( 85 % ), melting point m.p. 233 –
235 C , [9] .
Reflux a mixture of 1gm of the 2-amino -5-mercapto -1, 3,
4-thiadiazole with 3 gm of benzenesulphoyl chloride and 6
ml of pyridine for 30 min. pour the reaction mixture into
10ml of cold water and stir until the product crystallizes
.filter of the solid and recystallise it from ethanol. The
physical appearance, percentage of yield, melting point was listed in tablet 1[10].
The hydrated metal chloride salts of The Co(III), Fe(III), Cr(III), Ni(II) and Cu(II) (0.01 mol) was added to solution of the ligand (0.01mol) in hot absolute ethanol (40 mL) and the mixture was refluxed on a water bath for 2 hours and the solvent was evaporated in vacuum to half of the original volume and then cooled . The isolated complexes were filtered off , washed several times with ethanol and finally dried in air. The physical appearance, percentage of yield, melting point was listed in tablet 1 [11].
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015
ISSN 2229-5518
40
The purity of the ligand and its complexes were checked by TLC using silica gel-G as adsorbent.. Molecular formula, physical properties and Molecular weight and molar conductance data of the ligand and its complexes tabulated in table (1) and (2) .
elemental analysis ,Infra-Red Spectroscopy as shown in a figure (1,2) tabulated in table (3) and (5).The spectral data of
1H-NMR Spectra for the free ligand reported in figure (3)
while The Mass Spectra shown in a figure (4,5,6) and tabulated in Table(4). The calculated values were in a good agreement with the experimental values.
The FTIR spectrum for (L) 4-amino-N-(5-sulfanyl-1,3,4- thiadiazol-2-yl) benzene sulfonamide shows a characteristic stretching absorption bands . 3300 cm-, 3027cm-1, 2970 cm-1
,2760 cm-1,1635 cm-1 and 1480 cm-1, 1479 cm-1and 1339 cm-
1 ,assigned to υ N-H ,υC-H Aro, υC-H Ali, ʋ S-H, ʋ C=N of the thiadiazole ring, υS=O symmetrical C-S-C, asymmetrical C-S-C stretching , respectively. The C=N stretching vibrations are important to predict the bonding mode of the ligand ,these bands shift lower wavelength in the spectra of complexes compare with ligand, observed changes are the evidences of complexion had happened [13]. The IR data of the Ligand and complexes are shown in Table (5) and figure(1,2). The Characteristics groups exhibited by the ligands and complexes.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015
ISSN 2229-5518
41
The spectral data for the free ligand in DMSO-solution was reported along with the possible assignments in experimental. The proton nuclear magnetic resonance spectral data gave additional support for the composition of the ligand; all the protons are at their expected region. The Ar-H, NH ,NH2 and, SH proton signals, are shown in the regions of 7.2-8.2 ,5.8 ,5.2,3.8 ppm respectively, The number of protons calculated from integration curves and the recorded chemical shifts in figure (3)[12] .
The mass spectrum of the ligand shows a molecular ion peak [M/Z] at 288,the ligand spectra as shows fragment ion peak at m/Z (288,215,212,197,156,92,77 and 65 due to [C8H8N4O2S3]+. ,[C7H7N2O2S2]+., [C7H8N4O2S]+., [C7H7N3O2S]+.,
2
[C6H6NO2S] +., [C6H6N]+., [C6H5]+., [C5H5]+., respectively as
shown in Figure(4).The mass spectrum of the complex
[Co(L)2.Cl2]Cl shows a molecular ion peak at m/z 706 which
is equivalent to molecular mass of the complex. This complex shows another a fragment ion peak with loss of chlorine atom at m/z 671. the ligand spectra shows fragment ion peak with loss two chlorine atom at m/z (635,600) due to [Co(L)2Cl]+.and[Co(L)2]+. Respectively as shown in Figure (5) The mass spectrum of the complex [Fe(L)2.Cl2] +.Cl shows a molecular ion peak at m/z 703 which is equivalent to molecular mass of the complex. This complex shows another a fragment ion peak with loss of chlorine atom at m/z 668. the ligand spectra shows fragment ion peak with loss two chlorine atom at m/z (632,597) due to [Fe(L)2Cl]+.and [Fe(L)2]+. respectively .
The mass spectrum of the complex [Cr(L)2.Cl2] +.Cl shows a molecular ion peak at m/z 699 which is equivalent to molecular mass of the complex. This complex shows another a fragment ion peak with loss of chlorine atom at m/z 664. the ligand spectra shows fragment ion peak with loss two chlorine atom at m/z (628,593) due to[Cr(L)2Cl]+. and [Cr(L)2]+. Respectively.
The mass spectrum of the complex [Cu(L)Cl2]+.shows a
molecular ion peak at m/z 422 This complex shows another a fragment ion peak with loss two chlorine atom at m/z
387,351 due to [Cu(L)Cl ]+., [Cu(L)]+. Respectively as shown in Figure (6).
The mass spectrum of the complex [Ni(L)Cl2]+. shows a
molecular ion peak at m/z 417 , This complex shows another a fragment ion peak with loss two chlorine atom at m/z 382,347 due to [Ni(L)Cl ]+. , [Ni(L)]+. Respectively .
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015
ISSN 2229-5518
42
Table5. Infra-Red Spectroscopy absorption bands of ligand and its complexes | |||||||||
NO | Compound | υ N-H | υ C-H Ali Aro | ʋ S-H | υ C=N of ring υS=O | ʋ C-S-C Sy , Asy | υ M-N | υ M-O | υM-Cl |
1 | C8H8N4O2S3 | 3300 | 2970 3028 | 2760 | 1635 1480 | 1339 1479 | ------- | ----- | ------- |
2 | [Co(L)2Cl2]Cl | 3317 | 2980 3059 | 2721 | 1641 1481 | 1361Sy 1425Asy | 597 | 482 | 325 |
3 | [Fe(L)2Cl2]Cl | 3305 | 3031 | 2725 | 1630 1476 | 1324 1371 | 594 | 480 | 321 |
4 | [Cr(L)2Cl2]Cl | 3310 | 3040 | 2730 | 1618 1477 | 1354 1410 | 583 | 479 | 319 |
5 | [Cu(L)Cl2] | 3312 | 3047 | 2741 | 1622 1479 | 1340 1401 | 588 | 476 | 320 |
6 | [Ni(L)Cl2] | 3309 | 3050 | 2755 | 1625 1470 | 1372 1427 | 591 | 481 | 324 |
Analytical and spectra data (1H NMR,IR, mass spectra , elemental analyses C,H,N and Molar conductance of all
synthesized compounds were in full agreement with the
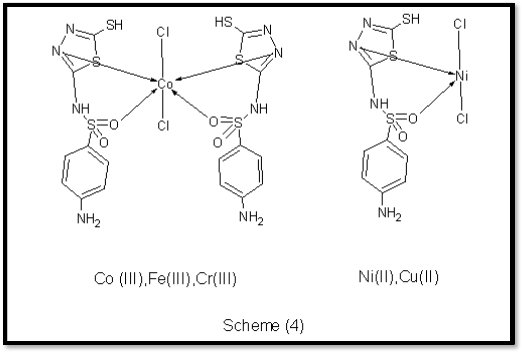
proposed structure . Based on the preceding discussion, the structure of the complexes suggested as follows in scheme (4)
below.
.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015
ISSN 2229-5518
43
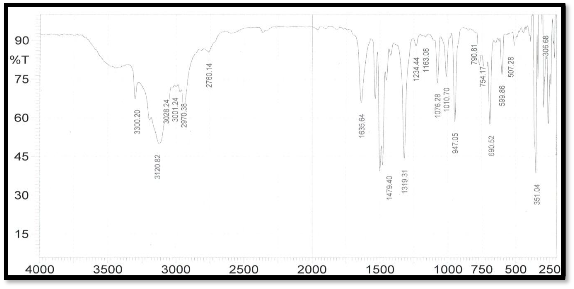
Figure (1) IR spectrum of the ligand cm-1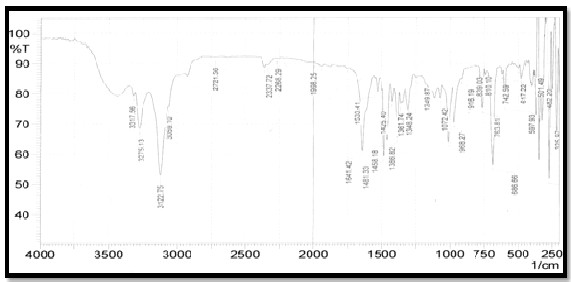
Figure (2) IR spectrum of the [Co(L) Cl ]Cl cm-1
2 2
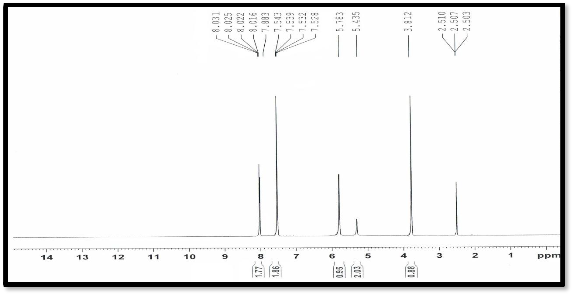
Figure (3 ) NMR spectra of the ligand
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015
ISSN 2229-5518
44
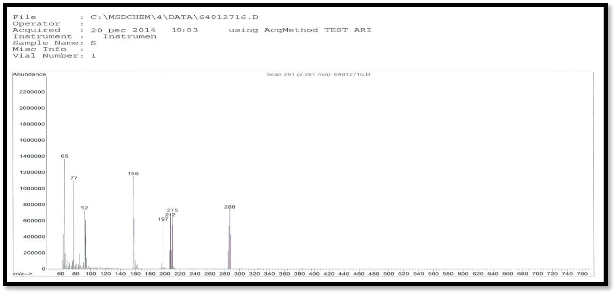
Figure (4) mass spectra of ligand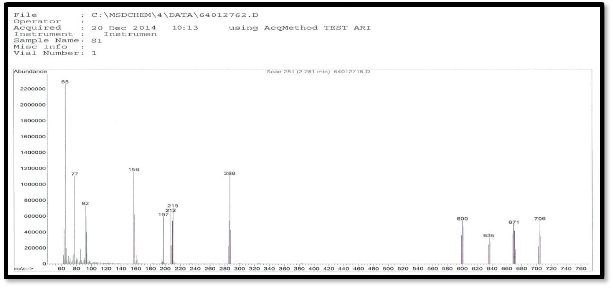
Figure (5) mass spectra of [Co(L)2Cl2]Cl cm-1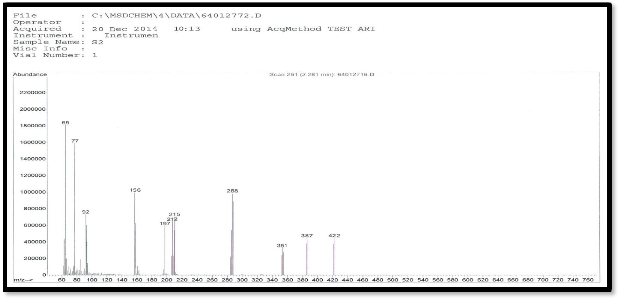
Figure (6) mass spectra of [Cu(L)2Cl2] cm-1
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015
ISSN 2229-5518
45
In the present work, a series of Co(III), Fe(III), Cr(III), Ni(II),
Cu(II) complexes with new ligand (L),have been prepared and characterized on the basis of IR,1HNMR, Mass spectroscopic as well as by elemental analyses C,H,N and Molar conductance.
According to all and the physiochemical measurements as the
prepared complexes, we can suggested the chemical configuration for the complexes. The ligand Synthesis of 4- amino-N-(5-sulfanyl-1,3,4-thiadiazol-2-yl) benzenesulfonamide was successfully synthesized. The ligand was treated to different transition metal salt to form the corresponding complexes as shown in the scheme (3). It may be concluded that the ligand coordinate through sulfonamide oxygen and thiadiazole nitrogen atoms.This view is further supported by the appearance of a band corresponding to the metal– nitrogen
, metal – oxygene stretching Vibration at 542–563 and 500-600
cm–1 respectively in the complexes . (Cr(III),Fe(III)and Co(III)
leading to the formation Octahedral geometry complexes
.while the Cu(II) and Ni(II) atoms leading to the formation
tetrahedral geometry complexes.
[1]Chemistry of synthesis drug J.Dharuman,2nd edition ,pp250 [2]-V.Alagarsamy ,Text book of medicinal chemistry ,Volume II,PP230,2002 .
[3]- Aziz-ur-Rehman, A. Siddiqa, M. A. Abbasi, S. Rasool, S. Z. Siddiqui, S. Gul,M. Ashraf and R. Nasar ; Enzyme Inhibition Studies on N-Substituted Sulfonamides Derived from m- phenetidine ; Pak. J. Chem. 3(3): 100-106, 2013 .
[4]-R.R Gupta,M.kumat,V.Gupta 2009, Heterocyclic Chemistry
Volume II five Membered Heterocyclic Page No 566 .
[5]- Jitendra Kumar Gupta*, Rakesh Kumar Yadav, Rupesh
Dudhe,Pramod Kumar Sharma ; Recent Advancements in the
Synthesis and Pharmacological Evaluation of Substituted 1, 3,
4-Thiadiazole Derivatives ; International Journal of PharmTech
Research ; Vol.2, No.2, pp 1493-1507, April-June 2010 .
[6]- Shakir Mahmood Alwan ; Synthesis and Preliminary
Antimicrobial Activities of New Arylideneamino-1,3,4- thiadiazole-(thio/dithio)-acetamido Cephalosporanic Acids ; molecules ; 2012, 17, 1025-1038 .
[7]- Sebastián Bellú, Estela Hure, Marcela Trapé and Marcela Rizzotto , THE INTERACTION BETWEEN MERCURY(II) AND SULFATHIAZOLE , Quim. Nova, Vol. 26, No. 2, 188-192,
2003 .
[8]ADRIANAHANGAN1*, ANDREEA BODOKI1, LUMINITA
OPREAN1,OVIDIUCRISAN2,ILISCAMIHALCA3;SYNTHESIS OFNEWN-SUBSTITUTEDHETEROCYCLICSULFONAMIDES; FARMACIA, 2012, Vol. 60, 6
[9]-Husam A. Ameen* and Ahlam J. Qasir ; Synthesis and
Preliminary Antimicrobial Study of 2-Amino-5- Mercapto
-1,3,4-Thiadiazole Derivatives , Iraqi J Pharm Sci, Vol.21(1) 2012. [10]- Vogel text book of practical organic chemistry fifthe edition pp 589.
[11]- KALAGOUDA GUDASI*, MANJULA PATIL, RAMESH VADAVI, RASHMI SHENOY and SIDDAPPA PATIL; Transition metal complexes with a new tridentate ligand , 5-_6-(5-mercapto-1,3,4-oxadiazol-2- yl)pyridin-2-yl_-1,3,4-oxadiazole-2-thiol. (2007) J. Serb. Chem. Soc.
72 (4) 357–366
[12] Rahmi Kasımoğulları1, Metin Bülbül1, Samet Mert1, and
Hülya Güleryüz , Synthesis of 5-amino-1,3,4-thiadiazole-2- sulphonamide derivatives and their inhibition effects on human carbonic anhydrase isozymes , Journal of Enzyme Inhibition and Medicinal Chemistry, 2011; 26(2): 231–237
[13] GOMATHI VELLAISWAMY* AND SELVAMEENA
RAMASWAMY, SYNTHESIS, SPECTRAL CHARA CTER IZ- ATION AND ANTIMICROBIAL SCREENING OF NOVEL SCHIFF BASES FROM SULFA DRUGS, International Journal of Pharmacy and Pharmaceutical Sciences, Vol 6, Issue 1, 487-
491 .
IJSER © 2015 http://www.ijser.org