International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 15
ISSN 2229-5518
Abstract-- Ni(II) and Mn(II) complexes were synthesized with m- hydroxy benzaldehyde semicarbazone (L1 = m-HBSC), m- hydroxy benzaldehyde thiosemicarbazone (L2 = m-HBTSC), p-hydroxy benzaldehyde semicarbazone (L3 =p-HBSC), p-hydroxy benzaldehyde thio-semicarbazone (L4 =p-HBTSC). The ligands were characterized on the basis of elemental analysis, IR, 1HNMR. While that of complexes were characterized by elemental analysis , molar conductance, magnetic moment, IR, electronic,
1HNMR and EPR spectral studies. On the basis , the complexes were found to have general composition [M(L)2 X2 ] (where M=Ni(II), Mn(II), L=L 1 ,L2 , L3 , L4 and X=Cl-,Br-,NCS-,SO4 2-). On the basis of IR, electronic and EPR spectra of complexes, octahedral geometries were found with planar coordination of ligand around metal ion and the anions occupies axial position.
Kew words – Semicarbazone and thiosemicarbazone, octahedral, Ni, Mn(II) complexes.
------------ ♦ -------------
The semicarbazone and thiosemicarbazone usually
behave as chelating ligands and usually react with metallic cations giving complexes.They are versatile ligands in both neutral and anionic forms. Metal complexes of semicarbazone and thio- semicarbazone have gained special attention due to their importance in medicine and biological system1.Nickel complexes occurred in several nickel-containing enzymes have been proposed to be involved in catalytic reaction2. Thiosemicarbazone are now well established as an important class of sulphur donor ligands
particularly for transition metal ion3-5. This is due to remarkable biological activities observed for
these compounds, which has since been shown to
be related to their metal complexing ability. Thiosemicarbazone derivatives exhibit a great variety of biological activities, such as antitumar6, antifungal7,8, antibacterial 8,9 and antiviral10. In the present work we synthesized Ni (II) and Mn(II) complexes of semicarbazone (m-HBSC)/(p-HBSC) and thiosemicarbazone (m-HBTSC)/(p-HBTSC)
————————————————
1. Department of Chemistry, M. M. H. College (C.C.S. University, Meerut), Ghaziabad-201009, India
E-mail: thakur.sapna61@gmail.com
2. Department of Chemistry,
Inderprastha Engineering College, Sahibabad, India
E-mail: nkschemistry71in@rediffmail.com
characterized through elemental analysis, IR,UV, and 1HNMR etc.
All the chemicals used were of Analytical R grade
and procured from sigma- Aldrich and Flucka. Metal salts were purchased from E. Merck and were used as received. All solvents obtained commercially were distilled before use.
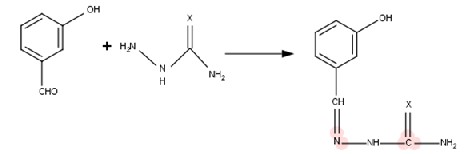
Hot ethanolic solution (50 ml) of, m-hydroxy benzaldehyde and p- hydroxy benzaldehyde(0.1
mol) was treated with ethanolic solution (50 ml)
of semicarbazide and thiosemicarbazide. The
resulting mixture was refluxed on water bath for
1-2 hour. On cooling the solution at 00C ,ligand is precipitated out. It was filtered and washed with
hot water and dried over P4 O10. For L1 ( m- HBSC) pale yellow long needles L2 (m-HBTSC) long, sharp yellow needles, L3 (p-HBSC) short whitish yellow needles, L4 (p-HBTSC)short dark yellow needles. The proposed structure can be shown according to the following reaction;
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 16

ISSN 2229-5518
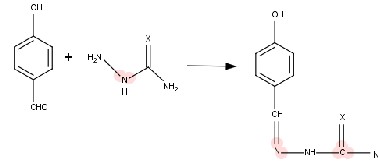
X =O, S in L1 and L2
X =O, S in L3 and L4
cm2 mol-1 thus the complexes [M(L2 ) 2 ]X 2 ] ( M= Ni
To a hot ethanolic solution (50ml) ligand L1 , L2 ,
L3 , L4 (0.1mol) dissolved in hot ethanolic
solution(50ml) of corresponding metal salt
(0.05mol) with mixed together with constant
stirring. Then mixture was refluxed for 4 hour
.On cooling a complex was precipitated out. The
complexes were filtered washed with, ethanol and dried in vacuum over P4 O10.
C, H and N were analysed on a Carlo Erba 1106 elemental analyzer. Molar conductance was
measured on the ELICO conductivity bridge. Magnetic susceptibility was measured at room
temperature on a Guoy balance using CuSO4 .5H2 O as calibrant. Infra-red spectra of ligand and their complexes have been recorded in KBr pellets on FTIR BX-11 spectrophotometer.
1H NMR spectra were recorded at room temperature on a Bruker Advance DPX-300
spectrophotometer using DMSO-d6 as a solvent. Electronic spectra of the complexes were recorded
in CHCl3 with a perkin Elmer lamda 15UV/V is spectrophotometer.
The molar conductance (M) of the complexes has been studied using DMF as solvent at the concentration of 10-3 we observed non- electrolytic behaviour of complexes and conductivity values lay in the range 12-18 Ohm-1
(II) and Mn(II), X= Cl-, Br-, NCS-, SO 4 2- ) (Table 1).
All the complexes have composition [Ni(ligand) 2 X 2 ] [X= Cl-,Br-, NCS-, SO4 2-] show magnetic behaviour of octahedral nickel(II) complexes relatively simple. Nickel (II) has the electronic configuration 3d8 and should exhibit a magnetic moment higher than expected for two unpaired electron in (2.8-3.2 BM) octahedral geometry.
All the complexes have composition have composition [Mn(ligand)2 X2 ] [X= Cl-,Br-,NCS-, SO4 2-] show a very good agreement with standard magnetic moment value 5.81 to 5.91 B.M. of complexes having five unpaired electrons at room temperature given in table. In the high spin octahedrally coordinated Mn2+ complexes, the lowest configuration(t 2g )3 (eg)2 gives rise to the ground states.
The reaction of ethanolic solution of m-HBSC/m- HBTSC, p-HBSC/p-HBTSC with metal salts gave complexes of the general formula [M(ligand) 2 X 2 ] where X = Cl-, Br-, NCS-, SO4 2-- respectively, as established on the basis of microanalysis and
conductance values.The general reaction can be expressed by the following equation.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 17
ISSN 2229-5518
MX2 .nH2 O + 2L ⟶ ML2 X2 + nH 2 O
Where L = L1 , L 2 , L3 ,L4 and M= Ni (ll)and Mn(II)
All the complexes are thermally and hydrolytically
stable and could be stored for several for several months, and most of them have sharp melting point. They are poorly soluble in water, ethanol and methanol and in other common organic solvents, but are soluble in DMF and DMSO.
A study and comparison of infra- red spectra of free ligands (m-HBSC), (m-HBTSC), (p-HBSC) and (p-HBTSC) and their metal complexes imply that all ligands behave as bidentate behave as metal is coordinated through nitrogen of azomethine group (Table-2).
due to 𝜈(>C=N), 1560 cm-1 is shifted towards
40 cm-1 coordination takes place through the nitrogen atom of imine groups and oxygen atom
of >C=O group. Thus it is implied that ligand L3
behave as bidentate.
1550 cm-1 due to 𝜈(>C=N) is shifted towards
lower side by 10-20 cm-1 on complexation,
coordination takes place through the nitrogen
atoms of imine group and the shifting 𝜈(>C=S)
805cm-1 towards lower side by 20-30 cm-1 suggest
involvement of sulphur in coordination. Thus, it is implied that ligand L 4 behaves as bidentate.
The ligands have been found to bidentate in
nature and coordination is confirmed by (M-O) at
520-460 cm1, 𝜈(M-N) at 420-390 cm-1 and 𝜈(M-Cl)
at 450-590 cm-1 vibration in L1 , L3 respectively14-
15. In thiosemicarbazone complexes ( L 2 , L 4 ) 𝜈(M-
N) at 450-465 cm-1and 380-395 cm-1 due to 𝜈(M-
lower side by 10-80 cm-1 on complexation11.
Indicate that the coordination takes place through the nitrogen atom of imine groups. The
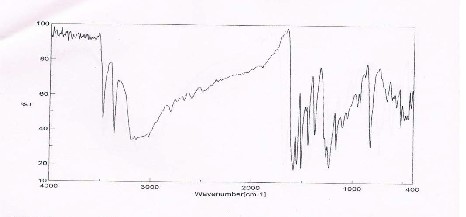
S)16,17. The IR spectrum of Ni(L4
fig.(1).
)Cl2
shown in
position of ligand band due to 𝜈 (>C=O), 1630
cm-1 is also shifted to lower wave number by 10-
40 cm-1 this indicate that the coordination takes
place through oxygen atom of >C=O group. Thus
it is implied that ligand L 1 behave as bidentate.
1510 cm-1 due to 𝜈(>C=N) is shifted towards
lower side by 10-20 cm-1 on complexation12. This
indicates that the coordination takes place through the nitrogen atoms of imine group and
the shifting 𝜈(>C=S) 780 cm-1 towards lower side
by 20-30 cm-1 suggest involvement of sulphur in
coordination. Thus, it is implied that ligand L2
behaves as bidentate.
due to 𝜈(>C=N), 1590 cm-1 is shifted towards
lower side by 10-80 cm-1 on complexation13. The
position of ligand band due to 𝜈 (>C=O), 1680
cm-1 is also shifted to lower wave number by 10-
groups are N bonded18 and are in similar
environment. A six coordinate structure with two bidentate semicarbazone /thiosemicarbazone compounds and two N- coordinated thiocyanate groups is suggested for these complexes.
IR spectra of these complexes show bands corresponding to bidentate sulphate group. For a bidentate sulphate group the symmetry is C2v and
each 𝜈R 3 and 𝜈R 4 band is split into three
components. In the complexes the four S-O
stretching bands are observed near at 1100-1108,
1090-1076, 1060-1065 and 980-990 cm-1 indicate
the bidentate nature of sulphate group19.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 18
ISSN 2229-5518
Figure 1: IR spectrum of Ni(L4 )2 Cl2
The Ni(II) complexes exhibits three bands in the regions,11700-11100 cm-1,18400-18100 cm-1 and
24700-24000 cm-1 which may be assigned to
3A2g(F) → 3T2g(F), 3A2g(F) → 3T1g(F),
3A2g(F) → 3T1g(P) transitions respectively, which
indicate octahedral20 geometry for all the Ni(II)
complexes,which is further supported21,22 by the
µeff value in the range 3.11-3.23 BM, for all the
multiplets between 𝛿 6.80 and 𝛿 7.70 ppm are all
due to the aromatic protons of phenyl rings.The
signals of the =N-NH protons were observed as
singlets at 𝛿 11.21-11.53. The signals of of the
HC=N protons which appears as singlet at 𝛿 8.03-
8.17 in the ligands show a shift to downfield in 𝛿
0.003-0.80 after complexation. This shift indicates
the coordination of imine nitrogen to the metal
23
Ni(II) complexes
centre
.The NH2 signal in the ligands L1 , L2 , L3 ,
Electronic spectra of the complexes display weak absorption bands in the range 9681-18635, 18450-
27100 cm-1, 21475-30860, 27300-34950 cm-1
which are characteristics of octahedral geometry.These bands may be assigned as 6A1g
L4 appear as doublets at 𝛿 7.98-8.45 due to the
non-equivalence of amine protons. Suggesting
the involvement of azomethine group in the bond formation. The broad band at 10.02 ppm in the
1H NMR spectrum of ligands assigned to OH
→ 4T2g (4G) 𝜈
,6A1g
→ 4Eg
, 4A1 g (4G)
proton, appear in the spectra of metal complexes
6 4 4
(10B+5C) 𝜈R 2 ,
A1g →
Eg( D) (17B+5C) 𝜈R 3 and
suggesting −OH group not upon coordination to
6A1g → 4T2g (4P)( 𝜈
respectively(Table 3)
) transitions
metal ion. The peaks around 3.5 and 2.5 are for
water and solvent i.e. DMSO respectively. The
1HNMR spectrum of ligand L2 and [Mn(L4 )Cl2 ]
shown in fig(2) and (3).
In the 1H NMR spectrum of semicarbazone
ligands and the thiosemicarbazone ligands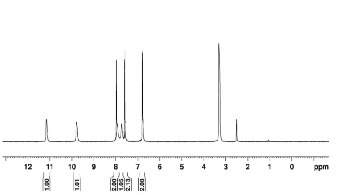
Figure 2: 1HNMR spectrum of ligand (L2 ) Figure 2:
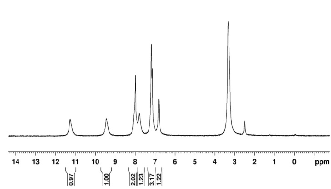
1HNMR spectrum of metal complex Mn(L4 )2 Cl2
The authors are thankful to IIT Delhi for recording
1HNMR and ARBRO Pharmaceutical LTD.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 19
ISSN 2229-5518
Analytical Division, Kirti Nagar, New Delhi for recording IR and UV spectra.
OC
Compounds | V(OH) | V(NH) | VC=O | VC=N | VC=S | VM-O | VM-S | VM-N |
L1 | 3335m | 3500-3000 | 1630 | 1460 | 350 | |||
L2 | 3332m | 3372-3100 | 1510 | 780 | 355 | |||
L3 | 3436m | 3270-3100 | 1650 | 1490 | 360 | |||
L4 | 3537m | 3273-3160 | 1592 | 680 | 355 | |||
L5 | 3437m | 3272-3180 | 1680 | 1432 | 362 | |||
L6 | 3556m | 3171-3165 | 1453 | 740 | 372 | |||
[Ni(L1 )2 Cl2 ] | 3500-3000 | 1621 | 1490 | 750 | 365 | |||
[Ni(L2 )2 Cl2 ] | 3500-3000 | 1491 | 750 | 405 | 350 | |||
[Ni(L3 )2 Cl2 ] | 3500-3000 | 1639 | 1493 | 460 | 350 | |||
[Ni(L4 )2 Cl2 ] | 3500-3000 | 1587 | 652 | 400 | 340 | |||
[Mn(L1 )2 Br 2 ] | 3200-3000 | 1660 | 1583 | 465 | 360 | |||
[Mn(L2 )2 Br 2 ] | 3270-3001 | 1584 | 720 | 410 | 350 | |||
[Mn(L3 )2 Br 2 ] | 3273-3000 | 1630 | 1581 | 462 | 350 | |||
[Ni(L4 )2 (NCS)2 ] | 3271-3000 | 1502 | 743 | 400 | 340 | |||
[Mn(L1 )2 (NCS)2 ] | 3261-3000 | 1632 | 1541 | 443 | 355 | |||
[Ni(L4 )2 SO4 ] | 3251-3000 | 1561 | 732 | 413 | 360 |

λmax(cm -1 )
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 20
ISSN 2229-5518
𝝂R1 | 𝝂R2 | 𝝂R3 | 𝝂R4 | ||
[Ni(L1 )2 Cl2 ] | 3.01 | 11300 | 18150 | 24700 | |
[Ni(L2 )2 Cl2 ] | 2.95 | 10100 | 18625 | 24000 | |
[Ni(L3 )2 Cl2 ] | 2.98 | 11320 | 1800 | 24300 | |
[Ni(L4 )2 Cl2 ] | 3.01 | 11500 | 20400 | 24000 | |
[Ni(L2 )2 (NCS)2 ] | 3.91 | 10681 | 1896 | 24300 | |
[Mn(L2 )2 Br 2 ] | 5.08 | 12520 | 20620 | 24020 | 28176 |
[Mn(L3 )2 Br 2 ] | 5.11 | 16600 | 18400 | 24000 | 30765 |
[Mn(L4 )2 Br 2 ] | 5.23 | 9681 | 18760 | 24600 | 30654 |
[Mn(L2 )2 SO4 ] | 5.34 | 9570 | 18720 | 24520 | 32178 |
Mn(L4 )2 (NCS)2 ] | 5.81 | 12000 | 18418 | 26708 | 34380 |
1. D.R. Kumar ;M. Ayesha, Res. J. Chem.
Environ, 15, 5 2011.
2. Stwertka A. guide to the elements. Revised ed.
Oxford University Press. New York, 94-95,
(1998).
3. Padhye SB, Kauffman GB. Transition metal complexes of semicarbazone and Thiosemicarbazone coordination chemistry Reviews 63: 127-160, (1985).
4. West DX, Padhye SB, Sonawane PB, Chikte RC. Structural and physical correlation in the biological properties of transition metal N- hetrocyclic thiosemicarbazone and S – alkyldithiocarbazate complexes structure & bonding 76: 1, (1991).
5. West DX, Padhye SB, Sonawane PB, Chikte RC. Copper(ll) complexes of tridentate (O,N,S) Thiosemicarbazone. Asian journal of chemistry Reviews 4(1):125,(1990).
6. A. G. Quiroga, j.M. Perez, I. Lopez-Solera, et al., “Novel tetranuclear orthometalated complexes of Pd(11) and Pt(11) derived from p-isopropylbenzaldehyde thiosemicarbazones with cytotoxic activity in cis-DDP resistant tumor cell lines. Interaction of these complexes with DNA,” Journal of medicinal chemistry, 41, 9, 1399-1408,(1998).
7. R. F.F. Costa, A. P. Rebolledo, T. Matencio,
et al., “Metal complexes of 2-benzoylpyridine- derived thiosemicarbazones structural, electrochemical and biological studies,” Journal of coordination chemistry, 58 ,15
,1307-1319,(2005).
8. R. K. Agarwal, L. Singh, and D. K. Sharma, “Synthesis, spectral, and biological properties of cooper(11) complexes of thiosemicarbazones of Schiff bases derived frim 4-aminoantipyridine and aromatic aldehydes,” Bioinorganic chemistry and Application, 59509, 10, (2006).
9. O. P. Pandey, S. K. Sengupta, M. K. Mishra, and C. M Tripathi, “Synthesis, spectral and
antibacterial studies of binuclear titanium(1V)/zirconium(1V) complexes of piperzine dithiosemicarbazones,” Bioinorganic chemistry and Application,1 (1) 35-44, (2003).
10. C. Shipman Jr., S.H. Smith, J.C. Drach, and D.
L. Klayman, “Thiosemicarbazones of 2-
acetylpyridine, s2-acetylquinoline, 1- acetylisoquinoline, and related compound as inhibitors of herpes simplex virus in vitro and in a cutaneous herpes guinea pig model,” Antiviral Research, 6(4), 197-222,(1986).
11. L.Latheef; M.R. Kurup, Spectrochim Acta
A,72,687, (2000).
12. S. M. Emam; F.A. E-Saied; S.A. Abou-Enein; H.A. El- Shater, Spectrochim, Acta, A,72,291, (2009).
13. S.Chandra; L.K. Gupta; Sangeetika,Spectrochim Acta A,62,453, (2005).
14. Frausto da Sliva, J.J.R.; Watton, R.; Gillard,
R.D .J. Chem. Soc, 3369,1970.
15. Kanoonga, N.; Singh, R.V.; Tanda J.P.J. Prakt.
Chem.Soc.3369,(1970).
16. West, D.X. Luckwood, M.A.; Spectral nature antifungal activity and molecular structure of metal complexes of acetyl pyridine 4N- substitututed Thiosemicarbazones. Transition Metal Chemistry 18, 221-227,(1993).
17. Ayman, K., El- Sawaf, West, D.X., Copper(II) complexes of 4-formyl antiopyrino N(4)- substituted thiosemicarbazones. Transition Metal chemistry 22,360-365, (1997).
18. Chandra, S.,Kumar, U., Spectral studies of
coordination compounds of cobalt (II) with thiosemicarbazones of heterocyclic ketones. Spectrochimica Acta Part A 62, 940-944., (2005).
19. Chandra, S., Kumar, U., Verma, H.S., Cobalt(II) complexes of semicarbazone and thiosemicarbazones. Journal of Saudi Chemical Society 7(3), 337-346 .
20. Krishna, C.H., Mahapatra, C.M. and Dash, A.K., J. Inorg. Nucl. Chem., 39,1253, (1977).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 21
ISSN 2229-5518
21. Figgis, B.N., Introduction to ligand field, Willey Eastern Ltd., New Delhi, 279, (1976).
22. Carlin, R.L.and Van Dryneveledt, A. J., Magnetic properties of transition metal comounds, Springer- Verlag, New York, 1997.
23. H. S. Seleem, A.A. Emara, and M.Shebl, “ The relationship between ligand structures and their CoII and Ni(II) complexes; “synthesis and characterization of novel dimeric Co II/CoIII complexes of bis (thiosemicarbazone),” Journal of Coordination Chemistry,58(9), 803-809, (2005).
IJSER © 2013 http://www.ijser.org