International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 553
ISSN 2229-5518
Poly (ethylene oxide)/Polyurethane based gel polymer electrolytes for lithium batteries
J. Shahitha Parveen and S.S.M. Abdul Majeed
ABSTRACT
A gel polymer electrolyte (GPE) based on an electrospun membrane of Poly (ethylene oxide) / Polyurethane (PEO/PU) incorporated with lithium ions was prepared and its performance in lithium batteries were studied. Blended form of PEO and PU electrospun fibrous membranes were prepared by electrospinning technique. Incorporation of PU in PEO decreased the crystallinity and the amorphous region enhances the mobility of ions.The presence of functional groups, thermal stability and morphology of the fibrous membranes were characterized by fourier transform infrared spectra (FT-IR), thermogravimetric analysis (TG) and scanning electron microscope (SEM).The
polymer blend electrolytes had the ionic conductivity in the order of 10-3 S cm -1.The experimental results show that the PEO/PU polymer
blend electrolyte prepared in this work would be a promising material for rechargeable lithium batteries.
Index Terms – Electrospun, Gel polymer electrolyte, Impedance, Ionic conductivity, lithium batteries, Poly (ethylene oxide), Polyurethane.
—————————— ——————————
1. INTRODUCTION
Recently, polymer electrolytes have received wide spread at-
in PEO was used to suppress the crystalinity and enhance ion-
IJSER
tention due to their technological uses in solid state electro-

chemical devices, rechargeable lithium batteries, fuel cells, super capacitors etc. [1,2]. The host polymers considered for the preparation of gel poymer electrolytes are polyvinylidene fluoride (PVdF), poly (ethylene oxide), poypoly(vinyl chlo- ride) (PVC), polyacrylonitrile (PAN), poly(vinyl pyrrolidone) (PVP), poly(vinyl sulfone) (PVS), etc [3-5]. Among the poly- mer hosts, poly (ethylene oxide) forms most stable complexes with inorganic salts and possess higher solvating power for salt than any other polymers [6, 7]. The main problem which restricts the use of PEO for polyelectrolytes is its semi – crys- talline nature. Crystalline portion of PEO hinders the migra- tion of lithium ion and hence PEO – based polyelectrolytes have low conductivity [8, 9]. Blending of polymers has proved
Shahitha Parveen.J, Assistant Professor Dept. of Polymer Engineering, B.S.Abdur Rahman University, Chennai. Mail Id: shahithaji@bsauniv.ac.in
S.S.M Abdul Majeed, Professor, Dept. of Polymer Engineering, B.S.Abdur
Rahman University, Chennai. Mail Id: majeedssm@bsauniv.ac.in
to be an effective route for improving the properties. [10, 11]. As polyurethane is more amorphous in nature while poly (ethylene oxide) is crystalline, blending of these two polymers
will act as a host for polymer electrolytes. The addition of PU
ic conductivity. Electrospinning is a novel and efficient tech-
nique for preparing microporous membranes that are com- posed of ultra fine fibers with diameters in the range of several micrometers to nanometers [12]. The PEO/PU electrospun nanofibrous membranes were prepared by electrospinning technique. Lithium salt was also incorporated into these blends (PEO/PU) to study the effect on ionic conductivity of the electrospun fibrous membrane.
2 .EXPERIMENTAL
2.1 Materials
Poly (ethylene oxide) (PEO), polyurethane (PU), dimethylfor- mamide (DMF), lithium hexafluorophosphate (LiPF6) , eth- ylene carbonate (EC), propylene carbonate (PC) were pur- chased from Sigma Aldrich and used as such.
2.2 Preparation of PEO/PU electrospun fibrous membrane
Fibrous membranes were prepared from 1g of PEO added to different concentration of PU (0.3, 0.4, 0.5g) and were dis- solved in a common solvent (Dimethylformamide=10ml) at 75
°C for 24h. The resulting homogeneous solution was electro- spunned to obtain nanofibrous membrane as described in pre-
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 554
ISSN 2229-5518

vious publications [13, 14]. The applied voltage was 17kV, tip to collector distance was 15 cm, flow rate was 0.25 ml/h and the rotating speed of the cylinder was 2000 rpm. The fibrous membranes were collected on a rotating cylinder roller wrapped by flat aluminium foil. The membranes were vacu- um dried at 60°C for 24h.
2.3 Electrochemical Impedance measurements of nanofibrous polymer electrolytes

The polymer electrolytes were prepared by immersing the electrospun PEO/PU fibrous membranes in an electrolyte so- lution of 1M Lithium hexafluorophosphate LiFP6 – EC/PC (1:1 volume) for 4 h in a glove box filled with argon gas [15, 16]. After the soaking time, the electrospun fibrous membrane was removed from the electrolyte solution and excess electrolyte solution was wiped off with a filter paper. Test cells were as- sembled by sandwiching polymer electrolyte between two stainless steel electrodes, SS/polymer electrolyte/SS (SS: stain- less steel) [12, 15]. The ionic conductivity was measured using electrochemical impedance spectroscopy (Won A Tech) over the frequency range of 1Hz to 100 KHz at an amplitude of
5mV.
3. RESULTS AND DISCUSSION
3.1 FT- IR spectroscopy study
The FT – IR spectra of pure PEO is shown in Fig.3a. The peaks at 2890, 1467, 1359, 1280, 844 cm-1 are assigned to C – H stretching, CH2 scissoring, CH2 wagging, CH2 twisting, CH2 rocking. The peaks at 1132, 1112 and 1062 cm-1 are assigned to C-O-C stretching vibrations .The semi crystalline phase of PEO is confirmed by the presence of triple peak at C-O-C stretching[21].
The FT-IR spectrum of pure PU in Fig 3b implies the
characteristic peaks of a strongly hydrogen bonded polyure- thane hard segment. PU showed characteristic absorption at
3338 cm-1 corresponding to urethane linkage (N-H stretching),
1701cm-1 corresponding to C=O stretching in urethane and
1536 cm -1 corresponding to N-H bending in urethane[22].
The overlaid spectrum of PEO and PEO/PU blend in Fig.3c was obtained by blending of PEO with 3%, 4% and 5% concentration of PU. The FTIR spectra of PEO /PU blend con- taining 3% of PU shows peaks at 3326 cm-1 and 1793 cm-1 as- cribed to N – H stretching and C=O stretching urethane link- age. Similiarly, the characteristic peaks at 3326 cm-1 and 18488 cm-1 was observed in the spectrum of PEO/ PU blend contain- ing 4% of PU and the peaks at 3789cm-1 and 1797 cm-1 was observed in the spectrum of PEO/PU blend containing 5% of PU , which confirms the urethane N – H stretching absorption and C=O stretching absorption. The appearance of new peaks along with changes in the existing peaks in the FTIR spectra
confirms the blending of PEO with PU.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 555
ISSN 2229-5518

3.2 Thermogravimetric Analysis
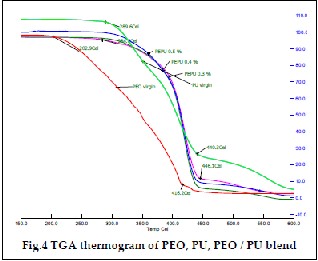
Thermograms of PEO, PU and PEO/PU polymer blend are shown in Fig.4. From the thermogram obtained for pure PEO,
3.3 Scanning Electron Microscopy
it was inferred that onset initial degradation at 196°C was due to the decomposition of the backbone of PEO. It was also ob- served that 95% of decomposition of PEO was at 452.7°C and has low char yield. PU has two stage weight losses starting at
296°C with 5% residual mass at 450°C [23]. The decomposition of PEO/PU bend followed a similar pattern in thermogram as that of pure PU. The PEO/PU blend containing 3%, 4% and
5% decomposes at 446°C, 441.9°C and 442.2°C. These results
indicate that PEO/PU blend has good thermal stability.
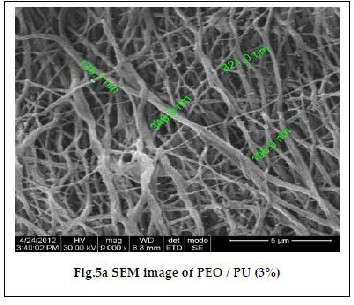
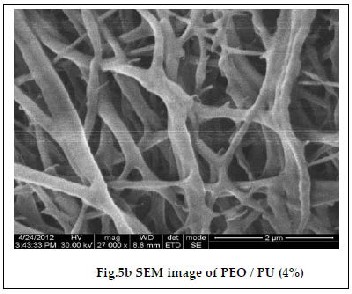
The morphology of the polymer electrolyte depends on the type of host polymer, solvent, preparative techniques as well as the evaporation rate of the solvent. These parameters influ- ence the electrochemical and physical properties of the electro- lyte [17]. Fig. 5a and 5b show SEM images of PEO/PU blend fibrous membrane comprising of a microporous structure composed of fully interconnected multi fibrous layers and interstices between ultra – fine fibers, with an approximate diameter of 100 – 320 nm [18]. The average fiber diameter was found to decrease due to the increase in electrospinning volt- age. The average fiber diameter of the fibers increased with
the increase in the polymer concentration due to the increase
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 556
ISSN 2229-5518
in the viscosities of the solution. The surface of the nano fibers was very smooth with well - controlled fiber diameter. All these fibers were collected over a cylindrical drum.This collec- tor can collect uniform fiber mats in large area and the diame- ter of the fiber was also stable.
3.4 Ionic Conductivity
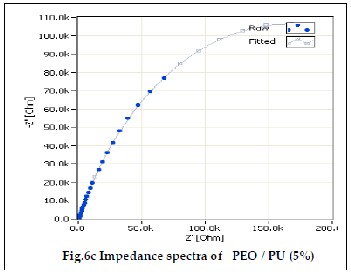
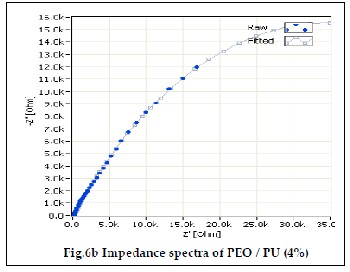
The electrochemical impedance spectroscopy is an excellent tool to characterize the electrochemical properties of the mate- rials and their interfaces with the electronically conductive electrodes [19]. Fig 6a, 6b and 6c shows the ionic conductivity of PEO/PU blend polymer electrolytes. The ionic conductivi- ties of the gel polymer electrolyte were measured using EIS method. In an impedance spectrum, also known as Cole – Cole plot, the real part of the impedance (Z’) was plotted against the imaginary part of the impedance (Z”) for data collected at various frequencies. The bulk resistance of polymer electrolyte was determined from the impedance spectrum.The ionic con- ductivity of the polymer electrolytes was calculated from the equation (1).
Where
= L/Rb * S (1)
– ionic conductivity of the gel polymer electrolyte
S – area of the electrolyte
L – thickness of the electrolyte
The pure PEO has a very low conductivity in the range of 10-7
Rb – Bulk resistance of the electrolyte
to 10-5
Scm-1
due to high degree of crystallinity [24]. The PEO-
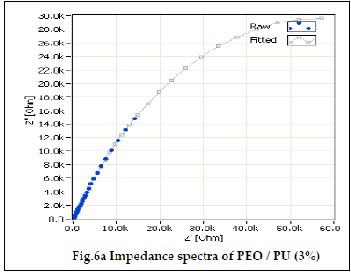
PU polymer blend containing 3%, 4% and 5% of PU show ion- ic conductivity value of 1.06607 10-3 x Scm-1, 1.22929 10-3 x Scm-1 and 3.1998410-3 x Scm-1. It can be observed that PEO/PU blend polyelectrolytes show higher ionic conductivity than pure PEO due to their electrolyte solution uptake. Because of the high porosity, interconnected cavities and high electrolyte uptake of polymer electrolyte membranes, the ions migrate and increase the ionic conductivity [25, 26]. The addition of amorphous PU lowered the crystallinity of PEO and promotes
the lithium ion mobility in the polyelectrolytes.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 557
ISSN 2229-5518
4. CONCLUSION
The polymer electrolytes based on fibrous PEO/PU blend membranes were prepared by electrospinning technique. FTIR spectrum confirms the miscibility of PU in PEO/PU blend fibrous membrane. The TG analysis reveals that PEO/PU blend polyelectrolytes have better thermal stability than pure PEO. SEM images showed that there was no bead formation in nanofibrous membrane. The fibrous membrane exhibits a po- rous morphology with average fiber diameter of 100 – 320 nm. The blending of PEO with PU has increased the conductivity and from a.c. impedance spectroscopy, it has been shown that the ionic conductivity of PEO/PU blend polyelectrolytes was in the range of 10-3 S/cm, found to be higher than pure PEO. Thus PEO/PU microporous electrolytes can be potential and promising candidates for lithium battery applications.
[7] A. Karmakar, A.Ghosh, Dielectric permittivity and electric modulus of polyethylene oxide (PEO) – LiClO 4 composite electrolytes, Current Applied Physics,12, pp 539- 543, 2012.
[8] Pier Paolo Prosini, Stefano Prosini, A lithium battery electrolyte based on gelled polyethylene, Solid state ionics, 146, pp 65 – 72, 2002.
[9] Zheng Zhong, Qi Cao, Bo Jing, Xianyou Wang, Xiaoyun Li, Huayang Deng, Electrospun PVdF- PVC nanofibrous polymer electrolytes for polymer lithium – ion batteries, Materials Science and Engineering B
177, pp 86-91, 2012.
[10] Mohammed Saleem Khan, Abdul Shankoor, Jan Nisar, Conductance study of poly (ethylene oxide ) – and poly (propylene oxide ) – based polyelectrolytes, Ionics, 16. pp 539 – 542, 2010.
[11] S. Rajendran, O. Mahendran, R. Kannan, Lithium ion conduction in plasticized PMMA – PVdF polymer blend electrolytes, Materials chemistry and physics, 74, pp 52 – 57, 2002.
[12] Jeong Rae Kim, Sung Won Choi, Seong Mu Jo, Wha Seop Lee, Byung Chul Kim, Electrospun PVdF – based fibrous polymer electrolytes for lithium ion polymer batteries, Electrochimica Acta 50, pp.69-70, 2004.
ACKNOWLEDGEMENT
[13] Zhizhen Zhao, Jingqing Li, Xiaoyan Yuan, Xiang Li, Yuanyuan
IJSEZhang, Jing ShReng, Preparation and properties of electrospun Poly-
We gratefully acknowledge Department of Science and Tech-
nology for providing financial support for this research work.
REFERENCES
[1] Anantha Iyenger Gopalan, Padmanabhan Santhosh, Kalayil Manian Manesh, Jin Hee Nho, Sang Ho Kim, Chul – Gyun Hwang, Kwang – Pill Lee, Devel- opment of Electrospun PVdF – PAN membrane – based Polymer electrolytes for Lithium batteries, Journal of Membrane Science, 325, pp. 683-690, 2008.
[2] Ji-Ae Choi, Yongku Kang, Hyojin Shim, Dong Wook Kim, Eunhee Cha, Dong – Won Kim, Cycling performance of a Lithium – ion pol- ymer cell assembled by in- situ chemical cross – linking with Fluori- nated phosphorous-based cross – linking agent, Journal of Power Sources, 195, pp. 6177-6181, 2010.
[3] A.M. Stephan, Review on gel polymer electrolytes for lithium batter- ies, Eur. Polym.Journal 42, pp. 21- 42, 2006.
[4] P.Santhosh, T. Vasudevan, A. Gopalan, K.P. Lee, Preparation and properties of new cross – linked polyurethane acrylate electrolytes for lithium batteries, Journal of Power Sources, 160, pp. 609-620,
2006.
[5] P. Santhosh, A. Gopalan, T. Vasudevan, K.P. Lee. Preparation and Characterisation of conducting poly(diphenylamine) entrapped pol- yurethane network electrolyte, J. Appl. Polym. Sci. 101, pp.611-617,
2006.
[6] A. Ghosh, P.Kofinas, PEO based block copolymer as solid state lithi- um battery electrolyte, ECS. Trans, 11, pp. 131-137, 2008.
vinylidene Fluoride Membranes, Journal of Applied Polymer Sci- ence,97,pp.466-474 , 2004.
[14] Sung Woo Lee, Sung Woo Choi, Seong Mu Jo, Byung Doo Chin, Dong Young Kim, Kwan Young Lee, Electrochemical Properties and cycle performance of electrospun poly (vinylidene fluoride) based fi- brous membrane electrolytes for Li – ion polymer battery, Journal of Power Sources, 163, pp. 41- 46, 2006.
[15] Yanhuai Ding, Ping Zhang, Zhilin Long, Yong Jiang, Fu Xu, Wei Di, The ionic conductivity and mechanical property of electrospun P(VdF – HFP)/PMMA membranes for lithium ion batteries, Journal of Membrane Science 329, pp. 56-59, 2009.
[16] Yasuo Matoba, Shohei Matsui, Masato Tabuchi, Takaaki Sakai, Elec- trochemical properties of composite polymer electrolyte applied to rechargeable lithium polymer battery, Journal of Power Sources, 137, pp. 284 – 287, 2004.
[17] Xin li, Gowrie Cheruvally, Jae – Won Choi, Jon Hyeon Ahn, Polymer electrolytes based on an electrospun Poly (vinylidene fluoride – co- hexafluoropropylene) membrane for lithium battery, Journal of Pow- er Sources, 167, pp. 491-498, 2007.
[18] Qizhen Xiao, Zhaohui Li, Deshu Gao, Hailiang Zhang, A novel sandwitched membranes as polymer electrolyte for application in li thium ion battery, Journal of Membrane Science, 326, pp. 260-264,
2009.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 558
ISSN 2229-5518
[19] Raghavan Prasanth, Vanchiappan Aravindan, Madhavi Srinivasan, Novel polymer electrolyte based on cob – web electrospun multi component polymer blend of polyacrylonitrile/ poly (methyl meth- acrylate )/polystyrene for lithium ion batteries – Preparation and electrochemical characterization, journal of Power Sources, 202, pp.
299 – 307, 2012.
[20] N.K. Chung, Y.D. Kwon, D.Kim, Thermal, mechanical, swelling, and electrochemical properties of poly(vinylidene fluoride) – co- hex- afluoroprpylene / poly (ethylene glycol) hybrid – type polymer elec- trolytes, Journal of Power Sources, 124, pp. 148 – 154, 2003.
[21] S.Noor.A.Ahmed.I.A. Talib, M.Y.A Rahman, Morphology, Chemical interaction and Conductivity of PEO-ENR50 based on solid polymer electrolyte, Ionics,16, pp. 161-170, 2010.
[22] C.V. Mythili, A Malar Retna, S. Gopalakrishnan, Synthesis, mechani- cal, thermal and chemical properties of polyurethanes based on car- danol, Bull. Mater Sci, 27, No. 3, pp. 235-241, 2004.
[23] Han-Hsin Kuo, Wei – Chih Chen, Ten – Chin Wen, A. Gopalan, A novel composite gel polymer electrolyte for rechargeable lithium bat- teries, Journal of Power Sources, 110, pp 27-33,2002.
[24] Pier Paolo Prosini, Stefano Passerini, A lithium battery electrolyte based on gelled polyethylene oxide, Solid state Ionics, 146, pp. 65-72,
2002.
[25] Zhiming Li, Feng Shan, Jiangong Wei, Jun Yang, Xinsong, Xinling Wang , High ionic conductive PVDF- based fibrous electrolytes, J Sol- id state Electrochem, 12, pp 1629 – 1635, 2008.
[26] Hao Li, Yue – Ming, Chen, Xiao – Ting Ma, Jun- Li Shi, Bao – Ku Zhu, Li – Ping Zhu, Gel polymer electrolytes based on active PVDF sepa- rator for lithium ion battery. 1: Preparation and property of PVDF/poly(dimethylsiloxane) blending membrane, Journal of Membrans Science, 379, pp. 397-402, 2011.
IJSER © 2013 http://www.ijser.org









