International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012 1
ISSN 2229-5518
1Assistant Professor,Department of Microbiology Acharya Nagarjuna University, Guntur
522510 A.P India.
2,3 Ph D Scholar,Department of Microbiology, Acharya Nagarjuna University, Guntur
522510 A.P India.
Abstract: Twenty three bacterial strains isolated from the soil of Chollangi mangrove environment and demonstrated Phosphate solubilization potential of isolated bacterial strains. Seven isolates showed phosphate solubilization activity. Morpho physiologically and biochemically identified two isolates (CMB1 and CMB2) as Bacillus subtilis, three isolates (CMFP3, CMFP4 and CMFP5) as fluorescent Pseudomonas and two isolates (CMAZ1 and CMAZ2) as Azotobacter sp. Plant growth promoting ability of seven bacterial strains tested for optimization of PH, carbon source and temperature and estimated phosphate solubilization quantitatively. Isolated strains visualized colonies at PH
8.6 after 72 hrs, where as pure cultures visualized early within 18 hrs at PH 6.8-7.2 range.
Observations indicated bacterial isolates of arid mangrove environment can easily adapted to normal soil PH and enhance phosphate content in soil.
Key Words: Azotobacter, Bacillus subtilis, Fluorescent Pseudomonas, Mangrove soil,
Phosphate solubilization.
Diverse microbial community
living in mangrove ecosystems continuously transforms nutrients from dead mangrove vegetation into sources of nitrogen, phosphorus, and other nutrients that can be used by the plants and in turn the plant-root exudates serve as a food source for the microbes. According to Forest Survey of India (FSI), mangrove wetland is 3, 48,710 ha .out of which nearly 56.7 % is present along the East Coast, 23.5 % along the West Coast and the remaining
19.8% in Andaman Nicobar islands.
Microbial biodiversity in mangrove ecosystem is one of the difficult areas of biodiversity research. Study of biogeography, community assembly and ecological processes in mangrove ecosystem require extensive
exploration, isolation and identification of potential microorganisms having specificity for recalcitrant compounds. Physical and chemical factors of mangrove ecosystem control the abundance and activities of bacteria in mangrove environment. Mangrove forests in India are productive ecosystems and sensitive to the environmental changes [1]. In the mangrove ecosystem, microorganisms perform various activities such as photosynthesis[2], nitrogen fixation[3], methanogenesis[4],agarolysis[5], production of antibiotics and enzymes etc., result in high productivity[6].
In the terrestrial environment, inoculation of insoluble phosphate solubilizing bacteria (IPSB) isolated from rhizosphere either alone or in combination increased the phosphate
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012 2
ISSN 2229-5518
content in soil and benefit crop plants like legume, sorghum and lettuce[6,7,8,9]. Where as in the marine environment IPSB isolated from rhizosphere of mangrove plants were reported to be potential for solubilization of insoluble calcium phosphate[10,11],but so far no report on potential phosphate solubilizing soil bacteria growing at different depths in mangrove environment . Therefore, presently an attempt was made to demonstrate the presence of insoluble phosphate solubilizing bacterial species from soil samples collected at a depth of 3 mts from Chollangi mangrove forest of East Coast to isolate, identify the species and to measure phosphate solubilizing potential invitro.
Soil sample were collected from mangrove environment of Chollangi of east coast at a depth of 3 ft and placed in zip locked plastic bags at 40 C .The soil contained 3.8% of organic matter and PH8.8.
1 gm of soil was separately suspended
in 9 ml of physiological saline soil in a flask and placed on an orbital shaker (at
100 rpm ) at room temperature(28±
20C) for 1 hr . At the end of shaking the soil samples were serially diluted upto 10-6 with physiological saline .
104-106 dilutions were placed on modified nutrient agar medium containing Flucanazole (antifungal antibiotic)by pour plate technique and incubated at 280C The most prominent colonies were isolated maintained on Nam slants at 40c for further
studies[12](Strickland and Parson
1972).
The protocol [13] was used for fluorescence production .Bacteria
were streaked on King’s B agar and incubated at 28± 20C for 48 h . at the end of incubation the plates were observed under UV light for production of fluorescence.
Each bacterial isolate was inoculated on the surface of Pikovskaya agar medium and phosphate solubilising activity was estimated after 1 to 5 days of incubation at room temperature phosphate solubilisation activity was determined by the development of the clear zone around bacterial colony.
Quantitative estimation of inorganic
phosphate solubilization was done as per methodology described by Nautiyal and Jackson .Bacterial isolates were grown in national Botanical Research Institute phosphate (NBRIP) broth containing 0.5% Tri calcium phosphate (TCP). The flask containing 50ml medium was inoculated with 500µl bacterial culture in triplicates and incubated at 30±0.1 at 180 rpm for 5 days in incubator shaker. Simultaneously the uninoculated control was kept under similar conditions. The cultures were harvested by centrifugation at 10,000 rpm for
10min. The phosphorus in supernatant
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012 3
ISSN 2229-5518
was estimated by vanado-molybdate- yellow color method. To a 0.5 ml aliquot of the supernatant , 2.5 ml Barton’s reagent was added and volume was made to 50 ml with de-inonized water. The absorbance of the resultant colour was read after 10 min at 430 nm in UV/Visible spectrophotometer. The total soluble phosphate was calculated from the regression equation for standard curve. The value of soluble phosphate liberated were expressed as
µg ml-1 over control. The PH of culture supernantant were also measured using a PH meter[13,14]
Twenty three bacterial
strains were isolated from mangrove soil of Chollangi ,East Godavari.The isolates were subjected to Phosphate solubilization and seven isolates were identified as phosphate solubilizers. These isolates were subjected to morphological,biochemical and physiological characterization with a view to identify them(Table1 and 2)
.The isolates CMB1 and CMB2 were positive to catalase and amylase where as negative to oxidase.CMFP3, CMFP4 and CMFP5 were positive to catalase
,amylase and oxidase.CMAZ1and CMAZ2 were catalase negative and positive to oxidase and amylase(Table
3)Fermentation of carbon components by the organisms was not identical and most of them were fermentative.(Table4)
Based on morpho physiological and biochemical characters,two isolates, CMB1 and CMB2 were identified as Bacillus sps
,three isolates CMFP3, CMFP4 and CMFP5 belong to the genus Pseudomonas sps and two other isolates namely CMAZ1and CMAZ2 belong to the Azotobacter sps.
Optimization of temperature, PHand carbon source were also studied . CMB1 ,CMB2 CMFP5 bacterial
isolates showed maximum growth at
40oC and CMFP3, CMFP4 , CMAZ1 and CMAZ2 Bacterial isolates at 45oC
.All isolates showed maximum growth at PH 8.8 in Mannitol medium and colonies were visualized after 72 hrs . Pure cultures showed maximum growth atPH6.8 in nutritive agar medium and showed visible colonies with in 18 hrs and may be due to
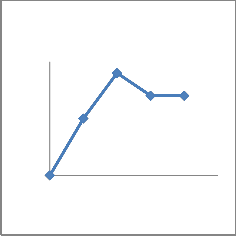
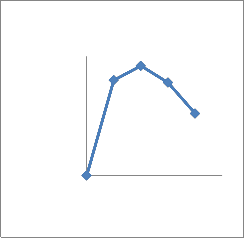
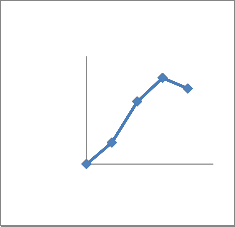
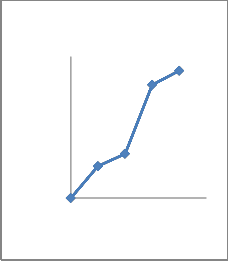
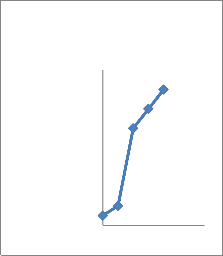
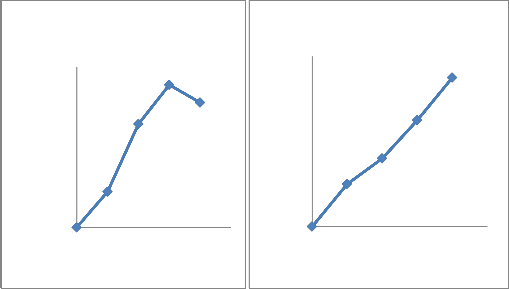
adaptation to osmotic stress. All the isolates were phosphate solubilizers. Quantitative analysis showed that the concentration of soluble phosphate increased abruptly with time and then decreased (Fig 2). All seven bacterial sps were capable of dissolving insoluble phosphate with different extent(Fig 2).The species with highest solubilizing capacity were CMFP3 and CMFP4(Fig 2 C,D),where as CMB1
And CMB2 dissolved moderately (Fig2
A,B) and CMAZ1 and CMAZ2 dissolved less. The increase in solubilization in all seven isolates was corresponding roughly to the logarithmic phase of growth of bacteria.Based on capacity to solubilise phosphate CMFP isolates were best and CMAZ were less potential.
The average concentration of dissolved
orthophosphates in seawater is
73µg/l[14] and concentration found in sediment of study site was 31 µg ml-
1and it was moderately higher than phosphorus of sea water,composed of insoluble phosphorus .VazQuez et al(2000) reported that fungi and IPSB present in the mangrove soil participate in releasing soluble phosphate into the pore water and may reach mangrove plant rhizosphere . The flow of nutrients like phosphorous between
sediment and water is complex phenomenon influenced by bacterial activitybecause of bacterial abundance(91%) of total microbial mass of mangrove soils[15]
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012 4
ISSN 2229-5518
Significant occurrence of PSB indicate that phosphotase enzyme from that group of bacteria plays crucial role in phosphorous cycling in the soil sediments of Mangrove forest[15]and also agood indicator of recycling of organic and iinorganic matter in mangrove environment IPSB bacillus strains solubilized 112-157mg/L of phosphate[7] and 0.5- 0.55 mg/l phosphate by marine sediment IPSB Vibrio sp and Pseudomonas sps[12].Our results of phosphate solubilizing activity are comparable to this study
Adaptation to osmotic stress was an exclusive character of moderately osmotic tolerant non halophylic microbes[16] (Rosenberg 1983). In Present investigation we are first time reporting that all seven ISPB isolates showed growth atPH6.8 in nutritive agar
medium and showed visible colonies with in 18 hrs may be an indicator of adaptation of PSB of mangrove soil growing under severely osmotic stress as halophylic microbes exhibiting exclusive character of terrestrial ISPB microorganisms.Extensive exploration identification ,isolation ,screening and use of this type of adaptable ISPB may speed up the development of mangrove plants for reforestration as well as plant
growth promotion in terrestrial crops like pulses, cereals etc.
Authors are thankful to UGC-New Delhi for financial assistants from the grant released for research project (F.No. 40-132/2011(SR)) and Central Instrumentation Centre of Acharya Nagatjuna University for laboratory facilities.
[1].K.Kathiresan..A review of studies on
Pichavaram mangrove, southeast
IndiaHydrobiologia 430. 2000 : 185–205. [2].K.Kathiresan and Bingham BL. Biology of mangroves and mangrove ecosystems. Adv Mar Biol 40,2001 :81-
251
[3].G.Toledo Bashan Yand Soeldner A
.Cyanobacteria and black mangroves in Northwestern Mexico: colonization,and diurnal and seasonal nitrogen fixation on aerial roots.Can J Microbiol 411995a,:999-
1011
[4].G.Toledo Bashan Y and, Soeldner A In vitro colonization and increase in nitrogen fixation of seedling roots of black mangrove inoculated by a filamentous cyanobacteria. Can J Microbiol
41,1995b,:1012-1020
[5].R.Mohanraju and Natarajan R. Methanogenic bacteria in mangrove sediments. Hydrobiologia:247,1992, :187-
93.
[6].B.R. Shome , R, Ahlawat SPS and Verma ND. Agar depolymerizing (agarolytic) bacteria isolated from mangrove soil samples of Andaman. Curr Sci 2000:79:696-97
[7].Sundara-Rao WVB, Sinha MK Phosphate dissolving micro-organisms in the soil and rhizosphere. Indian J Agric Sci
33,1963,,272–278
[8] R.Chabot H, Cescas MP Growth promotion of maize and lettuce by phosphate-solubilizing Rhizobium leguminosarum biovar phaseoli. Plant Soil
184,1996, :311–321
[9]S..Singh and , Kappor KK Effects of inoculation of phosphatesolubilizing microorganisms and an arbuscular mycorrhizal fungus on mungbean grown under natural soil conditions. Mycorrhiza
7,1998, :249–253
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012 5
ISSN 2229-5518
[9] P.A..Craven,and Hayasaka SS Inorganic phosphate solubilization by rhizosphere bacteria in a Zostera marina community.Can J Microbiol 28,1982,
:605–610
[10] RMN Kucey ,Phosphate-solubilizing bacteria and fungi in various cultivated and virgin Alberta soils. Can J Soil Sci
63,1983,:671–678
[11]. RMN…Kucey, Janzen HH and Leggett ME Microbially mediated increases in plant-available phosphorus. Adv Agron 42,1989,:199–228
[12]. C..Promod K and, Dhevendaran K. Studies on phosphobacteria in Cochin backwater. J Mar Biol Assoc India
29(1987):297–305
[13].P. Vazquez G. Holguin,, M.E. Puente A. Lopez-Cortes and Y. Bashan Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semi arid coastal lagoon Biol. Fertil Soils 30, 2000,:460–468
[14].N. Vassilev Solubilization of rock phosphate by immobilized Aspergillus niger. Biores Technol 59,1997:1–4
[15]. DM. Alongi .Bacterial productivity and microbial biomass in tropical mangrove sediments. Microb Ecol
15:1988, 59–79
[16].Rosenberg.A.Pseudomonas halodurance sp Nov a halo tolerant bacterium. Arch.Microbiolog 136,1983,
117-123
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012 6
ISSN 2229-5518
TABLE 1
MORPHOLOGICAL CHARECTERS OF BACTERIAL ISOLATES
FROM CHOLLANGI MANGROVE ENVIRONMENT
isolated strain | Morphological characters | |||
Gra m stain | Spore format ion | mot ile | pigme ntation | |
CMB1 | +ve | +ve | +ve | -ve |
CMB2 | +ve | +ve | +ve | -ve |
CMFP3 | -ve | -ve | +ve | +ve |
CMFP4 | -ve | -ve | +ve | +ve |
CMFP5 | -ve | -ve | +ve | +ve |
CMAZ1 | +ve | -ve | +ve | +ve |
CMAZ2 | +ve | -ve | +ve | -ve |
TABLE 2
BIOCHEMICAL ANALYSIS OF FOR BACTERIA ISOLATEDFROM CHOLLANGI MANGROVE ENVIRONMENT
Isoolate | BIOCHEMICAL TESTS | ||||||
I | MR | VP | C | G | H2S | NR | |
CMB1 | -Ve | -Ve | +ve | +ve | +ve | +ve | +ve |
CMB2 | -Ve | -Ve | +ve | +ve | +ve | +ve | +ve |
CMFP3 | -Ve | -Ve | -Ve | +ve | -Ve | +ve | +ve |
CMFP4 | -Ve | -Ve | -Ve | +ve | -Ve | +ve | +ve |
CMFP5 | -Ve | -Ve | -Ve | +ve | -Ve | +ve | +ve |
CMAZ1 | -Ve | +ve | +ve | +ve | -Ve | +ve | +ve |
CMAZ2 | -Ve | +ve | +ve | +ve | -Ve | +ve | +ve |
I-Indole, MR-Methyle Red, VP-Voges-Proskaver, C-Citrate, G Gelatin , H2S , NR-Nitrate reduction.
TABLE3
EXTRA CELLULAR ENZYMATIC ACTIVITIES OF BACTERIAL ISOLATED FROM CHOLLANGI MANGROVE ENVIRONMENT
Isolated srrain | Extra cellular enzyme activity | ||
Isolated srrain | C | O | A |
CMB1 | +ve | -ve | +ve |
CMB2 | +ve | -ve | +ve |
CMFP3 | +ve | +ve | +ve |
CMFP4 | +ve | +ve | +ve |
CMFP5 | +ve | +ve | +ve |
CMAZ1 | -ve | +ve | +ve |
CMAZ2 | -ve | +ve | +ve |
*C=catalase *O=oxidase *A=amylase
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012 7
ISSN 2229-5518
TABLE 4
ANALYSIS OF SUGAR FERMENTATION FOR BACTERIA ISOLATED FROM CHOLLANGI MANGROVE ENVIRONMENT
Isolated strain | Sugar fermentation | ||
Isolated strain | D | S | M |
CMB1 | +ve | +ve | +ve |
CMB2 | +ve | +ve | +ve |
CMFP3 | +ve | +ve | -ve |
CMFP4 | +ve | +ve | -ve |
CMFP5 | +ve | +ve | -ve |
CMAZ1 | +ve | +ve | +ve |
CMAZ2 | +ve | +ve | +ve |



*D=dextrose *S=Sucrose *M=mannitol
CMFP3 | CMFP4 | CMFP5 |
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012 8
ISSN 2229-5518
100
80
60
40
20
0
0 10 20 30
100
80
60
40
20
0
0 10 20 30
400
350
300
250
200
150
100
50
0
0 10 20 30
160
140
120
100
80
60
40
20
0
100
80
60
40
20
0
0 10 20 30
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012 9
ISSN 2229-5518
90
80
70
60
50
40
30
20
10
0
0 10 20 30
80
70
60
50
40
30
20
10
0
0 10 20 30
IJSER © 2012 http://www.ijser.org