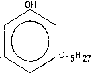International Journal of Scientific & Engineering Research Volume 2, Issue 4, April-2011 1
ISSN 2229-5518
Performance and Emission Characteristics of Stationary CI Engine with Cardnol Bio Fuel Blends
Mallikappa, Rana Pratap Reddy, Ch.S.N.Muthy
Abstract— The compression ignition engine is the most popularly used prime mover. The compression ignition (CI) engine moves a large portion of the world’s goods & generates electricity more economically than any other device in their size range [1]. All most all the CI engines use diesel as a fuel, but the diesel is one of the largest contributors to environmental pollution problems. The application of bio diesel as a substitute for conventional petroleum fuel in diesel engine gain ever increasing demand throughout the world wide, because it is produced from renewable resources, bio degradable and potential to exhaust emissions & use of bio diesel in diesel engines generates rural employ- ment opportunities by cultivating such oil producing crops[1-5]. In this research work the detailed investigation on performance and emission characteristics of four stroke single cylinder engine with variable loads were studied, cardnol bio fuel volumetric blends like 0, 10, 15, 20%, and 25% were used. The results indicate that brake power increases (by 76% approximately) as load increases. Brake specific energy con- version decreases (by 30-40 % approximately) with increase in load. Brake thermal efficiency increases with higher loads and emission levels (HC, CO, NOX) were nominal up to 20% blends.
Key words: Compression Ignition, characteristics, cardnol bio fuel, Performance, Emissions
Nomenclature
—————————— • ——————————
BSEC : Brake Specific Energy Consumption
B.T.E : Brake thermal efficiency B10 : Blend with 10%bio fuel CBF : Cardnol Bio Fuel
CI : Compression Ignition
CO : Carbon Monoxide
DR-CNSL: Double Refined Cashew nut Shell Liquid
EGT : Exhaust Gas Temperature
HC : Hydro Carbons
IC : Internal Combustion
NOx : Nitrogen oxide ppm : Parts per million Cs : Centistokes
1 INTRODUCTION
N today’s world the majority of automotive and trans- portation vehicles are powered by compression ignition engines. The compression ignition engine moves a large portion of the world’s goods & generates electricity more economically than any other device in their size range. All most all the CI engines use diesel as a fuel, but the diesel is one of the largest contributors to environ- mental pollution problems. Bio fuel is an alternative to petroleum based fuel, renewable energy source, bio de- gradable and non-toxic fuel, being beneficial for reser- voirs, lakes, marine life and other environmentally sensi- tive places such as large cities and mines & use of bio di- esel in diesel engines generates rural employment oppor-
tunities by cultivating such oil producing crops [1-5].
The issue of energy security led governments and re-
searchers to look for alternate means of renewable and
environment-friendly fuels. Bio fuel has been one of the
promising, and economically viable alternatives. Fuel and
energy crisis and the concern of society for depleting world’s non-renewable resources initiate various sectors to look for alternative fuels. One of the most promising fuel alternatives is the vegetable oils and their derivatives.
Plenty of scientific articles and research activities from around the world were printed and recorded. Oils from coconut, soy bean, sunflower, safflower, peanut, linseed and palm were used depending on what country they grow abundantly. It has been reported that in diesel en- gines; vegetable oils can be used as fuel, straight as well as in blends with the diesel. It is evident that [2] there are various problems associated with vegetable oils being used as fuel in compression ignition engines, mainly caused by their high viscosity. The high viscosity is due to the molecular mass and chemical structure of vegetable oils, which in turn leads the problems in pumping, com- bustion and atomization in the injector system of diesel engine. Due to the high viscosity, vegetable oils normally introduce the development of gumming, the formation of injector deposits, ring sticking as well as incompatibility with conventional lubricating oils in long-term opera- tions.
India is the largest producer, processor and exporter of Cashews, Anarcadium Occidentale Linn, in the world [6]. It was brought to India during the 1400 by Portuguese missionary. Cashew came conquered and took deep root
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 4, April-2011 2
ISSN 2229-5518
in the entire coastal region of India. While the tree is na- tive to central and Southern America it is now widely distributed throughout the tropics, particularly in many parts of Africa and Asia. In India Cashew nut cultivation now covers a total area of 0.70 million hectares of land, producing over 0.40 million metric tons of raw Cashew nuts. The Cashew (Anacardium Occidentale) is a tree in the flowering plant family Anacardiaceae. The plant is native to northeastern Brazil, where it is called by its Por- tuguese name Caju (the fruit) or Cajueiro (the tree). It is now widely grown in tropical climates for its cashew "nuts" and cashew apples.
1.1 Specification of Cashew nut shell
The shell is about 0.3 cm thick, having a soft feathery out- er skin and a thin hard inner skin. Between these skins is the honeycomb structure containing the phenolic material known as CNSL. Inside the shell is the kernel wrapped in a thin skin known as the teesta.
1.2 Composition of cashew nut
The shell is about 0.3 cm thick, having a soft feathery out- er skin and a thin hard inner skin. Between these skins is the honeycomb structure containing the phenolic material known as CNSL. Inside the shell is the kernel wrapped in a thin skin known as the testa.The nut consists of the fol- lowing kernel 20 to 25%, kernel liquid 20 to 25%, testa 2%, others rest being the shell. The raw material for the manu- facture of CNSL is the Cashew.

According to the invention [6] CNSL is subjected to fractional distillation at 200° to 240°C under reduced pressure not exceeding 5mm. mercury in the shortest possible time which gives a distillate containing cardol and the residual tarry matter, for example, in the case of a small quantity of oil, say 200 ml/ the distillation period is about 10 to 15 minutes. A semi-commercial or commercial scale distillation of CNSL may however take longer times. It has been found that there are certain difficulties of op- eration with regard to single-stage fractional distillation method, i.e. frothing of the oil which renders difficult the fractionation of cardol and also formation of polymerised resin. These difficulties can be over come in the two-stage distillation, if care is taken not to prolong the heating; this is to avoid the undue formation of polymerised resins and possible destruction partially or completely of the cardol or anacardol. When CNSL is distilled at a reduced pressure of about 2 to 2.5 mm. mercury, the distillate con- taining anacardol and cardol distils firstly at about 200°C to 240°C. This first distillate is then subjected to a second distillation under the same identical conditions of tem- perature and pressure when the anacardol distils over at a temperature of 205°C to 210°C and the cardol distils over at a temperature of 230°C to 235°C. In practice it has been found that the preliminary decarboxylation of the oil is essential, since there will be excessive frothing, which renders the distillation procedure unproductive and un- economical. A specific feature of this invention is that both cardol and anacardol may be obtained by a three- step process. The first step of the process is to get the de-
carboxylated oil by heating the oil to a temperature of
170°C to 175°C under reduced pressure of 30-40 mm.
mercury. The next two steps are the same as above for the
production of both cardol or cordnol and anacardol.
1.2.1Cardnol
DR-CNSL - Double Refined Cashew nut Shell Liquid. The Cashew Nut Shell Liquid (CNSL) obtained by pyrolysis. It mainly consists two naturally produced phenolic com- pounds: Anacardic acid 90% Cardol or cardnol 10%.

Cardnol obtained by pyrolysis from dr-csnl oil was uti- lized for testing purposes. Cardnol is a naturally occur- ring phenol manufactured from cnsl. It is a monohydrox- yl phenol having a long hydrocarbon chain in the Meta position.
C6H4 (OH)-(CH2)7-CH=CH-CH2-CH=CH (CH2)2 -CH3
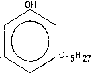
Reason for using the cardnol as alternative fuel: - it is renewable, it is cost effective, easily produced inexpen- sively in most regions of the world, results in reduced [up to certain extent] emissions compared with petro-diesels, results in no detrimental effects to the engine, non edible and it is extracted from the cashew nut shell not from the seed.
2 EXPERIMENTAL
The main objective was to study the performance and emission characteristics of the CI engine when Cardnol and pure diesel volumetric blends were used and also to investigate which combination of fuel blend is suitable for diesel engine at all load conditions from both perfor- mance and emission point of view. Experimentation has been conducted up to cardnol bio fuel volumetric blends like 0, 10, 15, 20%, and 25%, because the viscosities (refer table 1 for properties of cardnol bio fuel blends) of higher blends are more than the international standard limits [ASTM-allowable limits only up to 4-5 centistokes].
Properties Diesel B10 B15 B20 B25 B30
Flash point (C) 50 53 55 56 58 61
Density(Kg/m3) 817 823 829 836 841 846
Viscosity at
IJSER © 2014100C
http://www.ijser.org
(Centistokes)
Calorific value
(KJ/Kg)
2 2.5 3.1 3.5 4.2 5.5
40000 40130 40196 40261 40326 40392
International Journal of Scientific & Engineering Research Volume 2, Issue 4, April-2011 3
ISSN 2229-5518
2.2 Properties
Following table 1 indicates the properties of cardnol bio fuel blends. Lower Calorific value of the diesel has been considered for calculations. Table 1 Properties
2.3 Transesterification
Selection of raw materials: cardnol oil sample, anhydrous methyl alcohol 99% grade laboratory reagent type, So- dium Hydroxide was selected as the catalyst.
2.3.1 Procedure
About 4 grams of Naoh (catalyst) is dissolved in 200 ml methanol to prepare alkoxide, which is required to acti- vate the alcohol. Then stirring is done vigorously in a covered container until the alkali is dissolved completely for twenty minutes. Mixture is protected from atmos- phere carbon dioxide and moisture as both destroy the catalyst. The alcohol catalyst (Naoh) mixture is then transferred to the reactor containing 700 ml moisture free crude cardnol oil. Stirring of the mixture is continued for
90 minutes at a temperature between 60-65 degrees. The
round bottom flask was connected to a reactor condenser and the mixture was heated for approximately three hours.
2.3.2 Inference and observation
The mixture was distilling and condensing within the reactor Condenser, no glycerin, because CNSL is ex- tracted from honeycomb structure (shell) of a cashew nut. The color of cardnol oil slightly changed from dark brown to light brown color and an average of 95% recov- ery of bio fuel was possible.
2.4 Experimental procedure
The tests were conducted up to 25% blends, because the viscosity of above 25% blends exceeds the international standard limits (i.e. more than 5 Cs). The load test was conducted for different loads i.e. no load,
25%load,50%load, 75%load and full load conditions and
for blends such as 0%, 10%, 15%, 20%&25%of Cardnol.
The Orotech exhaust gas analyzer used for emission mea-

surements.
Fig 1 Experimental setup
In this investigation the various performance and emis- sion tests were conducted on four strokes single cylinder engine manufactured by M/s Kirloskar (as shown in
fig 1) Company limited. The parameter involved in per- formance analysis has been measured using the engine software supplied by the manufacturer.
2.4.1 Specifications of the engine
Name of the engine: KIRLOSKAR, TV1
General details: 4 stroke, C.I, Vertical
Type of cooling: Water cooled
Number of cylinders: 1
Bore: 87.5 mm
Stroke: 110mm
Rated power: 5.2 B.H.P at 1500 rpm
Dynamometer: Eddy current dynamometer
Compression ratio: 12:1 to 17.5:1
3. RESULTS AND DISCUSSIONS
The experiments were conducted on a direct injection compression ignition engine for various loads with an intention of studying the behavior of the engine in regard to various emissions, and performance characteristics when it was run on different volumetric blends and the results of the performance test and the emission studies conducted on the engine are plotted in the following (Characteristics graphs) figures.
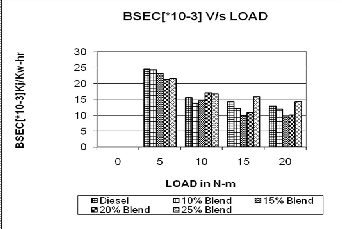
3.1 Brake specific Energy consumption
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 4, April-2011 4
ISSN 2229-5518
esel. This lower brake thermal efficiency obtained could be due to lower calorific value and increase in fuel consumption as compared to B20.
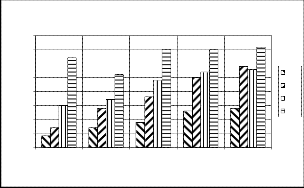
3.3 .Exhaust gas temperature & NOx Emissions
Exhaust gas temp.v/s Load
Fig 2.Brake specific Energy consumption
(*103Kj/Kw-hr) v/s load
Fig2.Depicts that, the brake specific energy consumption decreases by 30 to 40% approximately with increases in
800
700
600
500
400
300
200
100
0
0 100 200 300 400 500 600
Temperture in oC
B10Texh,NOx B15DTexh,NOx B20Texh,NOx B25Texh,NOx Dtexh,NOx
load conditions. This reverse trend was observed due to lower calorific value with increase in bio fuel percentage in the blends.
3.2 Brake thermal efficiency
Fig 4.Exhaust gas temp. v/s NOx Emissions
The variations of exhaust gas temperature and Nox emis- sions with respect to engine loading are presented in the in fig.4.The exhaust gas temperature increases linearly form 180o C at no load to 480 o C at full load conditions. This increasing trend of EGT is mainly because of gene- rating more power and consumptions of more fuel at higher loads.
3.4 HC Emissions
Load v/s HC Emissions
40
35
30
25
20
15
10
5
0
0 5 10 15 20
Load in N-m
B10
B15
B20
B25
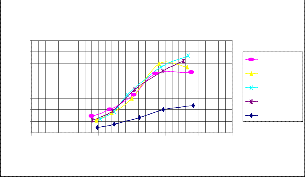
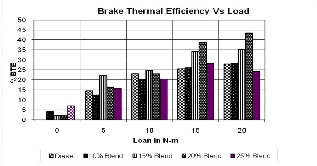
Fig 3.Brake thermal efficiency v/s Load
The variation of brake thermal efficiency with load for different volumetric blends is presented in fig.3.In all cas- es, it increased with increase in load. This was due to re- duction in heat losses and increase in brake power with increase in load. The maximum thermal efficiency for B20 (31%) was higher than that of the diesel. The brake ther- mal efficiency obtained for B25 was less than that of di-
Fig 5. HC Emissions
From the figure 5 it has been observed that HC emissions are nominal up to B20, and more at B25, the reason for this may be incomplete combustion.
3.5 Carbon Monoxide Emissions
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 4, April-2011 5
ISSN 2229-5518
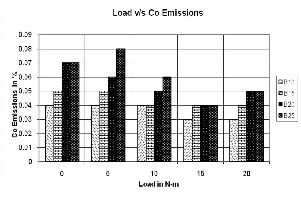
Fig 6. Carbon Monoxide Emissions
Carbon monoxide emissions increases with higher blends, and increases slightly more after 20% blends. The mini- mum and maximum Co produced was 0.03-0.08%.
At higher loads Co emissions slightly decreased. At ele- vated temperature, performance of the engine improved with relatively better burning of the fuel resulting in de- creased Co.
CONCLUSIONS
The cnsl and its extracts showed promising results in
terms of engine performance in par with conventional CI engine fuels. Based on the results of the study the follow- ing conclusions were drawn.
The significant factor of cardnol bio fuel is its low cost, its abundance and it is a byproduct of cashew nut industries.
The brake specific energy consumption decreases by 30 to 40% approximately with increases in load conditions. This reverse trend was observed dueto lower calorific value with increase in bio fuel percentage in the blends.
The brake thermal efficiency increases with higher loads. In all cases, it increased with increase in load. This was due to reduction in heat losses and increase in brake power with increase in load.The maximum thermal effi- ciency for B20 (31%) was higher than that of the diesel.
The brakethermal efficiency obtained for B25 was less than that of diesel. This lower brake thermal efficiency obtained could be due to lower calorific value and in- crease in fuel consumption as compared to B20.
The Nox emissions (ppm) increases with increasedpro- portion of blends and also with higher EGT. This trend mainly because of presence oxygen in bio fuel, this leads to more oxidation at higher temperature and responsible for more Nox emissions.
The HC emissions are nominal up to B20,and more at
B25,the reason for this is the incomplete combustion.
The Carbon monoxide emissions increases with higer blends, and increases slightly more after 20% blends. The minimum and maximum Co produced was 0.03-0.08%.At higherloadsCo emissions slightly decreased. At elevated temperature, performance of the engine improved with relatively better burning of the fuel resulting in decreased Co.
From this investigation it has been observed that up to20% blends of cardnol bio fuels may be used in CI engines without any modifications.
ACKNOWLEDGEMENTS
We are very much thankful to M/s Bangalore test house, Bangalore for testing various properties of cardnlo oil, & Dr.Sharanappa, Asst.Proff.of Mech.Engg.Dept, and Mr.Viswanath, Instructor R.I.T.M Bangalore, for their kind help during experimentation work. And special thanks Mr.Ganesh kamath of M/s Sanur Cashew nut In- dustries, Karkala for supplying CNSL to our experimen- tation work.
REFERENCES
[1] Alan C, Lloyd B, Thomas A. Cackette. “Diesel Engines: Environmental Impact and Control” California Air Resources Board, Sacramento, California ISSN1047-
3289 J. Air &Waste Manage. Assoc. 51:809-847
[2] Ayhan Demirbas “Studies on biodiesel from vegetable oils via transesterifications in supercritical Methanol” Energy Conversion and Management 44 (2003) 2093–2109
[3] Fernando Netoda Silvaa,*, Ant_onio Salgado Pratab,
Jorge Rocha Teixeiraca “Technical feasibilityassess ment of oleic sunflower methyl ester utilization in diesel bus engines.” Energy conversion and manage ment 44 (2003) 2857-2878
[4] K.Pramanik “Properties and use of jatropha curcas oil
and diesel fuel blends In CI engine,” Renewable Ener gy 28 (2003) 239–248
[5] N. Stalin and H .J. Prabhu “Performance test of IC en gine using Karanja bio diesel blendingwith diesel” ARPN Journal of engineering and applied science vol.2, no5, October 2007
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 4, April-2011 6
ISSN 222S-5518
[6] Piyali Das, T.Sreelatha, Anurada Ganesh "Bio oil from pyrolysis of cashew nut shell-characterization and related properties "Biomass and bio energy
IJSER ©2011 http /lwww l!ser org