Voidage ε = 1-(vc/vd) (1)
Where: Vc is the volume occupied of the fractionating height in the column, Vd is the volume of water displaced by 25cm height of column.
Flow through a packed bed can be described by the Ergun equation
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 1330
ISSN 2229-5518
Performance of a Plastic Packed Distillation
Column for Ethanol-Water Separation
Gideon Majiyebo Adogbo, Ayobiyi Joy Ayodele
Abstract - The performance of a distillation column was investigated using plastic packing materials of different shapes using binary mixture of water and ethanol at 80oC. The vapour generated was passed through three different polyethylene packing materials each to create resistance to the flow of vapour and provide the surface area that will facilitate mass transfer between the fluid phases. A hollow packing material A of 0.48 voidage,1.3cm particle diameter and a surface area of 72x10-6m2, created a pressure drop of 4.574N/m2, the product distribution of the distillate decreased from 87.5% to 75.5% by volume. A similar hollow material B with 0.54 voidage, 1.5cm particle diameter and a surface area of 128x10-6m2, created a pressure drop of
2.135N/m2, the product distribution of the distillate decreased from 81.25% to 53.13% by volume. A star shaped packing material C with 0.58 voidage,
2cm particle diameter and surface area of 117x10-6m2, it created a pressure drop of 0.885N/m2, the product distribution of ethanol decreased from
81.25% to 53.13% by volume. Plastic packings can be used in Ethanol-water distillation to reduce cost of production and energy consumption.
—————————— ——————————
1 INTRODUCTION
istillation is the most common process for separating large multi-component streams into high purity products which is carried out in columns. Trays and packing materials are used in distillation columns; here packing materials are used since they offer better separation and lower pressure drop across the column. In view of the technological challenges and increasing cost of packing materials, efforts to search for locally made materials that are cheap and available are being carried out, polyethylene packing materials are being used in this study, it is a widely used plastic. Conventional packed distillation columns always have some obstacles in selection of packing material and uniform distribution of fluids before they enter the packed bed [1], using hollow polyethylene packing material will help in overcoming this disadvantage since it is hard to dissolve in organic solvents and it maintains its performance for a long time due to resistance to oxidation by atmospheric oxygen, the hollow packings will provide a large amount of internal surface which will offer a higher flow resistance compared to the
external surface.
————————————————
Gideon Majiyebo Adogbo is currently a Doctor of Chemical Engineering in
Ahmadu Bello University, Nigeria.
Email: adogbogm@yahoo.com
Ayobiyi Joy Ayodele is a graduate of chemical Engineering Department, Ahmadu Bello University, Nigeria.
According to Yin et al.,[2], the liquid flow distribution is strongly affected by the packed bed height, liquid distributor design, packed bed height, gas flow rate and liquid viscosity. The geometric characteristic of the packing material affects the fluid flow distribution and contacting pattern of the fluid; as a result, different shape of polyethylene packing materials are used for the study to get the best geometric characteristic of the packing material. Guolito et al., [3] designed methods for a distillation column filled with a plastic structured packing, Guoliang et al., 2008 carried out a work using hollow fibres which has a better geometry of packing and realized a more productive distillation, saving more energy in production. several materials have been used as packings; [4], [5], [6], [7], [8] used different structured and random packing materials. Distillation is the dominant unit operation in the chemical process industry. Despite the classification of distillation as a mature technology, improvements in the design of contacting devices, especially packings, continue to be made [9]. The cost of obtaining these standard packings in Nigeria is very high thus the need to investigate local materials that can be used as packing materials in separation processes, this work aims at investigating the performance of distillation column using polyethylene packings of different shapes and comparing their efficiencies.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 1331
ISSN 2229-5518
The refractive index of pure distilled water and 96% ethanol were determined using the table refractometer. Thereafter different calculated volume mixtures of ethanol and water were made after which their refractive index were also determined. The determined values generated a data which was tabulated and used to establish a calibration plot. The refractive index of the initial boiling mixture and the collected products were deduced using the calibration plot.
300ml of ethanol water mixture with the concentration of
9.6% volume of ethanol was measured into a conical flask. The distillation column with the round bottom flask, Liebig condenser, still head, long bend receiver, condenser and beaker were setup. A chiller with a chilling temperature was connected to the lower end of the Liebig condenser and the other end to a container of water for recirculation, after which the chiller was switched on. Afterwards, the heating mantle was also switched on and was used to heat the mixture in the round bottom flask to boiling point; the distillation product was allowed to pass into the beaker at the end of the long bend receiver condenser. Care was taken to ensure that the temperature of the boiling mixture did not go beyond 80oC. The steady state temperature was maintained while the distillate was allowed to condense. After 56 minutes, the accumulated product in the condenser began to drop into the receiving flask. Drops of the distillate adding up to 2ml was collected and the time of collection was recorded. Seven other collections of 2ml was made and the composition of the desired more volatile component (ethanol) was determined using the table refractometer for each of the sample collected.
The same procedure as above was carried out using different packings. Packing A had a voidage of 0.48, packing B had a voidage of 0.53 and packing C had a voidage of 0.58 to create a pressure drop across the column with varying time.
From the work carried out, the following results were obtained:
TABLE 1: PARTICLE DIAMETER, VOIDAGE, PRESSURE DROP, AND SURFACE AREAS OF PACKING MATERIALS.
Packing A | Packing B | Packing C | |
dp (m) | 1.30x10-2 | 1.5x10-2 | 2.0x10-2 |
ε | 0.48 | 0.53 | 0.58 |
∆p(N/m) | 4.574 | 2.135 | 0.885 |
A(m2) | 72x10-6 | 128x10-6 | 117x10-6 |
Where (dp) is particle diameter, ε is the voidage, ∆p(N/m) is the pressure drop created within the column by the packing materials and A is the total surface areas of the packings.
Voidage ε = 1-(vc/vd) (1)
Where: Vc is the volume occupied of the fractionating height in the column, Vd is the volume of water displaced by 25cm height of column.
Flow through a packed bed can be described by the Ergun equation![]()
![]()
![]()
= + (2)
With the pressure drop, L = the height of the bed,
= the fluid viscosity, = the void space of the bed, uo = the fluid superficial velocity
dp = the particle diameter,
p = the density of the fluid
TABLE 2: DISTILLATION DATA USING THE FREE COLUMN WITH A VOLUME OF 2CM3.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 1332
ISSN 2229-5518
The table above shows the data obtained during the simple distillation of 9.8% ethanol composition by volume of
ethanol water mixture for eight readings. The values were collected for average time change of 60 seconds during which a fixed volume of 2ml of the distillate product was drained from the receiver. The percentage composition of the product obtained decreased from 62.5% and 43.7%, also the velocity of the fluid through the column varied between
0.0102 and 0.0108cm/s. The lighter (lower-boiling) i.e.
ethanol components tend to concentrate in the vapour phase, while the heavier (higher-boiling) components tend toward the liquid phase. The result is a vapour phase that becomes richer in light components as it passes up the column and a liquid phase that becomes richer in heavy components. The separation is the effect of the difference in the volatility of the ethanol and water in the boiling mixture. The percentage composition of ethanol decreased as the time progressed, because during the process, the vapour formed on boiling the liquid mixture is removed at once from the system. Since the vapour is richer in the more volatile component than the liquid, it follows that the liquid remaining becomes steadily weaker in the volatile component, thus, whilst the vapour formed over a short period is in equilibrium with the liquid, the total vapour is not in equilibrium with the residual liquid.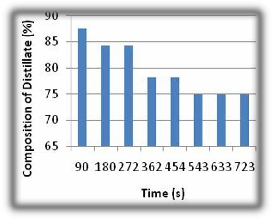
Fig 1 Distillation using Packing A in a Packed Column.
The Fig. 1 above shows the data obtained when the distillation column was packed with hollow packings having a voidage of 0.48, particle diameter of 0.138cm and a surface area of 72x10-6m2.
The product distribution of the distillate varied between
87.5% and 75.5%. The pressure drop across the column was
4.574N/m2. The volume of the distillate product of 2ml was
also collected. The packing material provided a large surface area to facilitate mass transfer between phases; it
also provided paths for liquid drainage and resulted in a low pressure drop for vapour. The collecting times were higher value than that obtained during simple distillation. This was due to the fact that the packing created resistance to the flow passing through the column, so the flow of the fluid was always delayed through the column. The performance of the packing was largely due to the pressure drop created within the column by the packing, the surface area available for mass transfer between the two phases- vapour and the condensed vapour of the fluid. The hollow nature of the packing material having a large amount of internal surface offered a higher flow resistance than its external surface [10]. Owing to incomplete wetting of the packing surfaces and to the formation of areas of stagnation in the liquid film, the effective area normally was significantly less than the total external area of the packing pieces, thus the available surface area did not facilitate separation beyond 87.5% of ethanol.
It is advantageous to have a pressure drop that will create a reasonable hold-up in the column as this promotes interphase contact [10], therefore the pressure drop created within the column was sufficient to provide effective mass transfer between the fluid phases but performance did not go beyond 87.5%. The condensed vapour distributed over the entire surface of the packing, flowed in thin films over the entire packing surface in the column. The films grew thicker along the walls and some portion of the column was covered by a stagnant film of condensed fluid. The localized path of the fluid proved vital.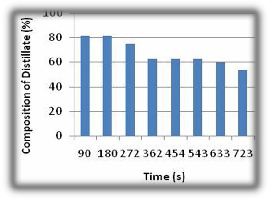
Fig 2. Distillation using Packing
This shows the data obtained when a different packing of
1.5cm particle diameter, 0.54 voidage and surface area of
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 1333
ISSN 2229-5518
128x10-6m2. The pressure drop during this separation was
2.135N/m2. The percentage composition of the distillate
decreased from 81.25% to 53.13%. The drop in the concentration of the distillate was sharper than that obtained in the Fig. 1; this could be due to channeling that was prominent in this case. Secondly there was less resistance to the flow of the less volatile component of the mixture due to low pressure drop. Also the voidage was higher creating more too much empty space in the column. However entrainments of condensed vapour within created different film layers within the column thereby reducing the available area for mass transfer between the fluid phases.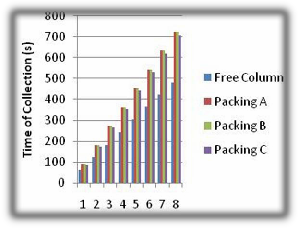
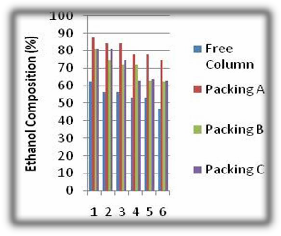
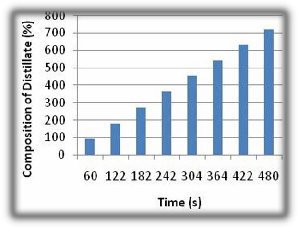 Fig 4. Ethanol Composition from Distillation
Fig 4. Ethanol Composition from Distillation
Fig 3. Distillation using Packing C
The packing used is star shaped having a diameter of
0.2cm, 0.58 voidage and surface area of 117x10-6m2. The composition of ethanol decreased from 81.25% to 53.13%.
This packing material has the lowest pressure drop with a value of 0.885N/m2. Low pressure is used in distillation to minimize the amount of energy used to cause the vapour to rise through the column. This Pressure drop ensured that less energy was used during distillation using the packing material although did not allow a good contacting pattern between the fluid phases. The amount of ethanol collected was low due to shape of the packings which gave poor contact between the two phases.
Fig 5. Time of Product Collection.
From the figure above, the free column has the lowest composition of ethanol collected as a result of no proper interfacial area for vapour-liquid contact, the collection time was also low since there was no resistance to flow and pressure drop which is created by packings. The provision of a packing with a high surface area per unit volume may not result in good contact of vapour and condensed vapour unless the liquid is distributed over the packing materials. Column packing performs better at low flow rates when the pressure drop is just low enough to maintain a reasonable liquid hold up in a column. The performance of the packing C was less than packing A because the pressure drop created within the column was not sufficient to create a reasonable hold up to ensure a more effective separation of the more volatile component from the boiling mixture, the
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 1334
ISSN 2229-5518
collection time using packing C was low compared to the other packings because the voidage of the packing was
large which did not allow proper contact between the two phases due to low effective area of contact. This is because of stagnant pools where liquid reaches saturation and no longer participates in the transfer process, the performance of packing B was higher than C but lower than packing A which is due to it having a larger voidage. The packings A gave more ethanol yield which was collected at longer time due to resistance created by the packings.
Using different shapes of packing material shows that the fluid dynamics and mass transfer are affected by the geometry and structure of materials in the column. The ethanol product collected for the packed columns were larger compared to that collected by the free column. The polyethylene packings all gave positive results but packing
‘A’ used in the distillation of ethanol gave a higher product distribution which ranged from 87.5% to 75.5% by volume thus polyethylene packing ‘A’ design can be used industrially for distillation especially in developing economies to reduce cost of production and the amount of energy that would be consumed during the process.
[1] Z. Guoliang, L. Lan, M. Qin and X. Youyi, “Separation of Alcohol–Water Solutions by Distillation through Hollow Fibers” Desaltination (223) Elsevier Science,
2008, 417-426.
[2] F. Yin, Z. Wang, A. Afacan, K. Nandakumar, and K.T
Chuang, “Experimental studies of liquid flow misdistribution in a random packed column” The Canadian Journal of Chemical Engineering, 78, 2000,
449–457.
“Design Method for Distillation Columns Filled with Metallic, Ceramic, or Plastic Structured Packings” Industrial and Engineering Chemistry Research, 1997
1747–1757.
[4] A.E Orlando, L.C Medina, M.F Mendes and E.M.A
Nicolaiewsky, “HETP Evaluation of Structured Packing Distillation Column” Brazilian Journal of Chemical Engineering, 26 (3), 2009, 619-633.
[5] D.C Lorenzo, Z. Olujić and P. Alessandro,
“Comprehensive Mass Transfer Model for Distillation
Columns Equipped with Structured Packings”
Industrial and Engineering Chemistry Research, 45 (23), 2006, 7967-7976.
[6] H. Verschoof and Z. Olujic, “A General Correlation for Predicting the Loading Point of Corrugated Sheet Structured Packings” Industrial and Engineering Chemistry Research, 1999, 3663-3669.
[7] C. Hatice and O. Canan, “Dynamic modeling and
Optimal Control of a Multicomponent Batch Packed Distillation Column” The International Federation of Automatic Control Seoul, korea, July 6-11, 2008, 4548-
4553.
[8] Y. Dali, M. Ronald, N.F Babak and W. Ronald,
“Performance characteristics of a new structured packing” Journal of Membrane Science 362, 2010, 86-
96.
[9] C.E Schmit, D. Cartel and R.B Eldridge, “Process
Tomography: An Option for the Enhancement of
Packed Vapour- Liquid Contactor Model
Development” Ind. Eng. Chem. Res. 39, 2000, 1546-
1553.
[10] J.M Coulson and J.F Richardson, “Chemical
Engineering” Fifth Edition, 2, Elsevier, New York,
2002.
IJSER © 2013 http://www.ijser.org
Internatio nal Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013
ISSN 2229-5518
1335
IJSER<S>2013 http /lwww f!ser org