International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 240
ISSN 2229-5518
Performance Characteristics of a DI Diesel Engine Fuelled With The Blends Of Diesel-Biodiesel- Ethanol
R. SenthilKumar1, R.Manimaran2, R. Ramadoss3
1Research Scholar, Mechanical Engineering, Annamalai University
2Research Scholar, Mechanical Engineering, Pondicherry Engineering College
3Assistant Professor, Mechanical Engineering, EGS Pillai Engineering College, Nagapattinam
t her mal s ent hi l 12@gmai l . c om
Abstract
Biodiesel (fatty acid methyl esters), which isderived from triglycerides by transesterification with methanol, has attracted considerable attention during the past decade as a renewable, biodegradable, and nontoxic fuel. Several processes for biodiesel fuel production have been developed, among which transesterification process by using alkali catalyst has been industrially accepted for its high conversion and short reaction times. However this type of catalyst is not able to be reused and requires tedious washing and separating steps. Recently, enzymatic transesterification has attracted much attention for biodiesel production asit produces high purity product and enables easy separation from the byproduct, glycerol. The high costsof lipase enzyme and lipase inactivation caused by methanol are the main obstacles for a commercially feasible enzymatic production of biodiesel fuels. In the present work to overcome the above mentioned problems, lipase enzyme is produced from Pseudomonasfluorescence culture and immobilized with sodiumalginate by using entrapment method for cost reduction and a solvent (n-hexane) is used for reduction in inactivation caused by methanol. The transesterification reaction was carried out in a batch reactor and the maximum yield of 92 % was obtained at 24 h at 40°Cwith a reaction mixture containing 6:1 molar ratio ofmethanol to substrate, 5g of enzyme and suitableamount of n-hexane based on molar ratio. Finally thephysical properties of D70B25E5(contains
70%diesel,25%biodiesel, 5%ethanol), D70B20E10(contains70%diesel, 20%biodiesel, 10%ethanol), andD100(contains pure diesel) were
analyzed and tested in asingle cylinder, four stroke, direct injection, constantspeed, compression ignition diesel engine (Kirloskar) to evaluate the performance characteristics.
Keywords—Biodiesel, transesterification, Immobilized lipase, pseudomonas fluorescence, blends.
I. INTRODUCTION
More than half of the world’s energy needs are supplied through petrochemical resources, such as coal and natural gases, with the exception of hydroelectricity and nuclear energy. All of these resources are limited and at current usage rates will be consumed shortly [1]. The rapid industrialization and motorization of the world has led to a steep rise for the demand of petroleum products. These reserves are highly concentrated in certain regions of the world. Those countries which are not having these reserves are facing a foreign exchange crisis, due to the import of crude oil. Now the world is currently facing the worst energy crisis in history. Most of the countries worldwide are still strongly dependent on petroleum resources as their main source of electricity and transportation fuel. Its price has been setting record highs in recent days. Hence, the one and only solution to this crisis is to find a sustainable (nonconventional) and economically practicable source of alternative energy. There are many alternative (nonconventional) energy sources such as wind, solar, geothermal, OTEC (Ocean Thermal Energy Conversion) and biomass which fulfill the first criterion (sustainability). But a few of these can fulfill the second criterion (economic practicability). Therefore, the best option, fulfilling the above, two criterions, is bio-fuel, particularly that are made readily available from biomass feed stocks [2]. The bio-fuel obtained from vegetable oils holds good promises as an eco-friendly alternative to petrochemical (or) diesel
fuels. One of such alternative fuels is triglycerides (vegetable oils /animal fats) on and their derivatives. Vegetable oils being renewable are widely available from a variety of sources and have low sulphur contents close to zero [3]. The useof 100% pure vegetable oil or animal fats to power diesel engines has several draw backs such as high fuel viscosity, low power output, thickening or gelling of lubricating oil and low volatility resulting in carbon deposits due to incomplete combustion [4]. Hence, vegetable oils are processed so as to acquire properties (volatility and viscosity) similar to that of fossil fuels [5]. Although, various processing techniques like Pyrolysis, micro emulsification and transesterification are available to convert vegetableoil to fuel form, transesterification is the most popular method of producing biodiesel. Currently biodiesel is most commonly prepared by alkali/acid catalyzed transesterification of an oil or fat with an alcohol, oracid catalyzed esterification when the feedstock has high free fatty acid content [6]. Chemical methods give high conversion ratio of triacylglycerols (TAG) to methyl esters (Biodiesel) in short times (4–10 h).
However, chemical transesterification are connected with some drawbacks as for example, high energy consumption and difficulty in the recovery of glycerol and high amount of alkaline waste water from the catalyst [7]. Enzyme catalyzed transesterification of oil is a good alternative to overcome these drawbacks.There are many reports on biodiesel production using enzyme
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 241
ISSN 2229-5518
catalysis by free or immobilized lipases.Immobilized lipase in particular is suitable for continuous biodiesel production because of the ease of its recovery from the reaction mixture [8]. Biocatalyst based fatty acid methyl ester formation is critically influenced by some process parameters, like enzyme concentration, molar ratio of oil to alcohol, reaction temperature, reaction time , pH and amount of solvent [9, 10]. Optimization of these parameters is likely to enhance the yield of the biodiesel product. There are two major limitations of lipase- catalyzed biodiesel synthesis. One is higher cost which can be reduced upto a certain extent by immobilization and another is its inactivation by methanol [11].However high operational stability of the enzyme was reported in several studies making its recycle possible in a batch system, or its long use in a continuous one, which reduces the incidence of catalyst cost [12, 13]. Among the possible raw materials for the production of biodiesel in India, cotton seed oil must be considered due to local marketing problems and easy availability.The selected alcohol for the synthesis would be methanol because of its low cost. The enzymatic methanolysis of cottonseed oil was studied recently.However, in that study, a large quantity of enzyme was necessary to obtain reasonable biodiesel yields [14]. Short chain alcohols, especially methanol, have low solubility in oils therefore a new liquid phase appears in the system at moderate concentrations leading to an inactivation of the enzyme and decreased yields of ester. This problem was overcome by the stepwise addition of methanol, since the solubility of methanol in the alkyl esters is greater than in the oil, and consequently limits enzyme deactivation [12, 15] and by using solvents like1,4dioxane, petroleum ether, tert -butanol, n-hexane etc.[16,17,18]. We found that when using n-hexane asa solvent the enzymatic process is improved, n-hexane dissolves both methanol and the glycerol and is not a substrate for the lipases because it does not act on tertiary alcohols. Moreover n-hexane relative low cost. The main objective of this experimental study isto determine the optimum conditions for the production of cottonseed oil methyl ester (biodiesel) with the low cost immobilized lipase enzyme, using n hexane as solvent, and performance studies of cottonseed oil methyl ester blends with pure diesel and ethanol in DI diesel engine.
2. Materials and methods
Cottonseed oil was purchased commercially from oil mills, Tamilnadu, India. It has the following characteristic: iodine value 114 mg I2/100 g, acid value
0.6 mg KOH/g and saponification value 199 mgKOH/g. The molecular weight of the cottonseed oil can be
calculated from its saponification and acid values, and was 840. Chemicals such as sodiumalginate, methanol, calcium chloride and hexane were of analytical grade purchased from Nice and Himedia Chemicals Pvt.Ltd. India. Pseudomonas fluorescence culture was obtained from microbial type culture collection and gene bank Chandigarh (India). The bioethanol (95%) pure used in these tests was kindly supplied by Himedia chemicals and diesel fuel was obtained from local commercial market. Culture maintenance, inoculums preparation etc. methods are to be carried out to extract lipase enzyme.
2.1 Lipase and its immobilization
Lipases are widely employed to catalyze hydrolysis, alcoholysis, esterification and transesterification of carboxylic esters. Lipases have excellent catalytic activity and stability in non aqueous media, which facilitate the esterification and transesterification process during biodiesel production. Immobilized enzymes are defined as “ enzymes physically confined or localized in a certain defined region of space with retention of their catalytic activities, and which can be used repeatedly and continuously”. There are several methods for lipase immobilization, including adsorption, covalent bonding, entrapment, encapsulation, and cross linking. These immobilization methods have been employed to improve lipase stability for biodiesel production. In the present work, Entrapment method is used for immobilizing the lipase. Entrapment of a lipase entails capture of the lipase within a matrix of polymer. In theory the entrapped enzyme is not attached to the polymer; its free diffusion is merely restrained. Virtues of the entrapment method for immobilizing lipase are that it is fast, cheap, and very easy and usually involves mild conditions [19].
2.2 Methanolysis of Cottonseed oil
Methanolysis reactions were conducted at stoichiometric molar ratio of oil/methanol (1:6); oiland methanol were poured into the reaction flask and heated to the reaction temperature with constant shaking using reciprocal shaker (180 revolutions/min) for 24 h. In subsequent experiments, in which the effect of molar ratio of oil/methanol was investigated, the volume of oil is kept constant and the volume of methanol is varied. Around
12 ml of hexane is added to the reaction mixture to increase the solubility of the reactants. The appropriate amount of immobilized whole cells based on oil weight was added to the flask. After 24 h of reaction time, the reaction was stopped and the cells were removed from the reaction mixture by filtration. The produced ester
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 242
ISSN 2229-5518
and byproduct glycerol were separated using separate funnel.
2.3Stability of immobilized lipase:
After the trans esterification reaction, the immobilized enzymes were separated from the reaction medium by filtration and submitted to different treatments before being reused. The treatments were performed by washing with different solvents. The solvents were n- hexane, propanol, ethanol, and water. The enzymes were washed with these solvents and after that dried for 24 h at 40 0˚C.From our study it was found that the washed immobilized lipase was suitable for five batch operations. Produced biodiesel was blended with diesel fuel and ethanol and its properties are shown intable 1.
Table 1.Fuel properties:
Properties | Diesel | D70B25E5 | D70B20E10 |
Diesel (% vol) | 100 | 70 | 70 |
Biodiesel (%vol) | 0 | 25 | 20 |
Ethanol (%vol) | 0 | 5 | 10 |
Viscosity (mm2/sec) at 40°C | 3.24 | 4.21 | 3.52 |
Density (Kg/m3) | 840 | 870 | 850 |
Calorific value (MJ/Kg) | 42.5 | 39.8 | 41.43 |
Catane number | 54.6 | 45.8 | 45.2 |
2.4 Experimental Set up and Procedure:
The performance tests were conducted on avertical, water cooled, single cylinder, four stroke, direct injection KIRLOSKER TV- I engine. The basic specifications of a test engine is given in table 1.The engine has been coupled with an eddy current dynamometer for applying different load conditions.The engine has always been run at its rated speed. The fuel injection system consists of three-hole type injector with a MICO plunger pump of 8mm diameter operated by the camshaft. The injection timing recommended by the manufacturer is 23° before TDC (static). The operating pressure of the nozzle has been set at the rated value of
220 kgf/cm2. The governor of the engine was used to control the engine speed. The engine had a combustion chamber with overhead valves operated through push
rods. Cooling of the engine is accomplished by supplying water through the jackets on the engine block and cylinder head.Provision has been made in the cylinder head surface to mount a piezoelectric pressure transducer for measuring the cylinder pressure. The emissions from engine smoke density and NOx, have been measured with the help of the AVL Di gas analyzer. A schematic diagram of the experimental setup is shownin Fig.1.The engine was started with petroleum diesel fuel and warmed up for a sufficient time in order to reach steady state operational conditions for each fuel.The results evaluated here were obtained at constant speed conditions at different engine brake horse power. For every fuel change, the fuel tank and lines were cleaned. Before running the engine to a new fuel, it was allowed to run for some time to consume the remaining fuel from the previous experiment.
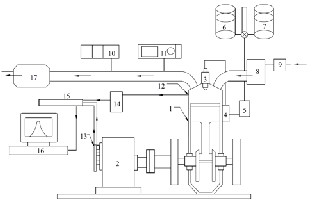
Figure 1: Schematic diagram of experimental set up
1. kirlsokar TV1 engine 10. AVL Smoke meter
2. Eddy current dynamometer 11. AVL DI-gas analyzer
3. Injector 12. Pressure transducer
4. Fuel pump 13. TDC Encoder
5. Fuel filter 14 .Charge amplifier
6. Diesel tank 15. .Indimeter
7. fuel tank 16. . Monitor
8. Air stabilizing tank 17. . Exhaust silencer
9. . Air filter
Table 2.Engine specifications
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 243
ISSN 2229-5518
40 D100 D70B25E5 D70B20E10
30
20
3. Results and discussion
3.1 Specific Fuel consumption:
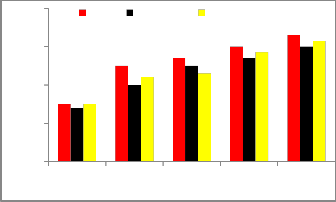
The variation of specific fuel consumption (SFC) with the brake power of diesel fuel and blends of D70B25E5, D70B20E10 is shown in figure 3. It is observed that blends of Diesel-Biodiesel Ethanol have higher SFC than diesel fuel. The higher specific fuel consumption is due to the lower energy content of the biodiesel blend and lower calorific value. It is clear from the Figure that as the load increases, the BSFC decreases for all fuels. Finally from the figure it was observed that the specific fuel consumption for the blend of D70B20E10 is close to that of diesel fuel
10
0
1 2 3 4 5
Brake power (kw)
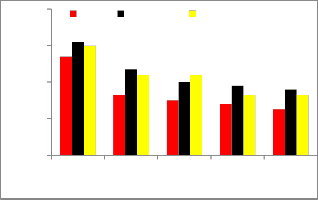
Fig 3.Brake thermal efficiency
3.3 Exhaust gas temperature:
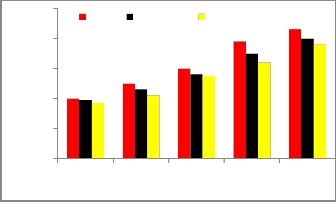
Figure-4 shows the exhaust gas temperature for the pure diesel fuel, and diesel-biodiesel-ethanol blend fuels for various brake horse power. It is observed that for all bio fuel blends, the temperature is very slightly lower than for pure diesel operation.This is due to the higher latent
heat of evaporation of the ethanol blends compared with
0.8
D100 D70B25E5 D70B20E10
that for the diesel fuel.
0.6
0.4
0.2
0
1 2 3 4 5
Brake power (kw)
Fig 2.Brake specific fuel consumption
500
400
300
200
100
0
D100 D70B25E5 D70B20E10
1 2 3 4 5
Brake power (kw)
3.2 Brake thermal efficiency:
Figure shows the variation of brake thermal efficiency versus brake power for pure diesel and diesel-biodiesel- ethanol blends. From figure it has been observed that the brake thermal efficiency isincreases with the increase in brake power of pure diesel and both oxygenated fuel blends. There is slight reduction in brake thermal efficiency for biodiesel blend was due to higher viscosity, higher volatility and lower calorific value [20].
Fig 4.Exhaust gas temperature
4. Conclusion
Cottonseed oil methyl esters were produced by enzymatic Trans esterification method and the biodiesel obtained was studied for fuel properties.Experiments were conducted on a single cylinder,four stroke, Water cooled, direct injection, diesel engine using cotton seed oil methyl ester blend and diesel as a fuel.The main results can be obtained as follows,
5.References
1. Production of cottonseed oil methyl ester as biodiesel was carried out using an immobilized Pseudomonas
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 244
ISSN 2229-5518
fluorescence lipase, and the critical process parameters were optimized for maximum conversion inbatch mode.
2. The optimized process based on the immobilized lipase as catalyst, resulted in a maximum conversion of
92%. The immobilized lipase also proved to be stable
and lost little activity when was subjected to repeated uses.
3. Blends of Diesel-Biodiesel Ethanol have higher SFC than diesel fuel. The higher specific fuel consumption is due to the lower energy content of the biodiesel blend and lower calorific value.
4. The brake thermal efficiency is increases with the increase in brake power of pure diesel and both oxygenated fuel blends.There is slight reduction in brake thermal efficiency for both biodiesel blends was due to higher viscosity, higher volatility and lower calorific value.
5. For both bio fuel blends the exhaust gas temperature is very slightly lower than for pure diesel operation. This due to the higher latent heat of evaporation of the ethanol blends compared with that for the diesel fuel.
REFERENCES
[1] Meher LC, Sagar DV, Naik SN. Technical aspects of biodiesel production by transesterification-a review. Renew Sustain Energy Rev 2006; 10: 248–68.
[2] N.N.A.N.Yusuf, S.K.Kamarudin, Z. Yaakub. Overview on the current trends in biodiesel production. Energy Conversion and Management 2011; 52: 2741-
2751.
[3]Barnwal BK, Sharma MP. Prospects of Biodiesel production from vegetable oils in India. Renew Sust Energy Rev 2005;9(4):363–78.
[4].A.S. Ramadhas , S. Jayaraj, C. Muraleedharan, Use of vegetable oils as I.C. engine fuel -A review, Renewable Energy, 29 (2004) 727–742.
[5]Jike Lu, KailiNie, FengXie, Fang Wang, TianweiTan, Enzymatic synthesis of fatty acid methyl esters from lard with immobilized Candida sp. 99-125,Process Biochemistry, 42 (2007)1367–1370]
[6].Srivathsan Vembanur Ranganathan, Srinivasan Lakshmi Narasimhan, Karuppan Muthukumar, An overview of enzymatic production of biodiesel, Bio resource Technology, 99 (2008) 3975–3981.
[7]J.M. Marchetti_, V.U. Miguel, Possible methods for biodiesel production. Renewable and Sustainable Energy Reviews 11 (2007) 1300–1311.
[8]Md Mahabubur Rahman Talukder, Enhanced enzymatic transesterification of palm oil to biodiesel. Biochemical Engineering Journal 55 (2011) 119–122.
[9] Alan C, Lloyd B, Thomas A. Cackette, ”Diesel Engines” Environmental Impact and Control” California Air Resources Board, Sacramento, California ISSN14047-3289 J. Air & Waste Manage. Assoc. 51:
809-847.
[10] Ayhan Demirbas “studies on biodiesel from vegetable oils via transesterifications in supercritical M ethanol” Energy Conversion and Management 44 (2003)
2093- 2109.
[11] Fernando Netoda Silvaa, Ant_onio Salgado pratab, J ore Rocha Teixeiraca “Technical feasibility assess ment of oleic sunflower methyl ester utilization in diesel bus engines.”Energy conversion and management 44 (2003) 2857-2878.
[12] K. Pramanik “Properties and use of Jatropha curcas oil and diesel fuel blends in CI engine,” Renewable energy 28 (2003) 239-248.
[13] N. Stalin and H.J. Prabhu “Performance test of IC engine using Karanja Bio diesel blending with diesel” A RPN journal of Engineering and applied science vol.2., no. 4 October 2007.
[14] Piyali Das, T. Sreelatha, Anurada Ganesh “Bio oil from pyrolysis of cashew nut shell-characterization and related properties ‘Biomass and bio energy.
[15] jamil Ghojel, Damon Honnery. Heat release model for the combustion of diesel oil emulsions in DI diesel engines. Applied Thermal Engineering 25 (2005). 2072-
2085.
[16] O.M.I. Nwafor & G.Rice. Performance of Rapeseed
Oil blends Engine Applied Energy.Vol.54,NO.4,pp.345-
354,1996.
[17] T.Ganapathy, K. Murugesan, R.P. Gakkhar, Performance optimization of Jatropha biodiesel engine model using Taguchi approach Applied Energy (2009).
[18] Murugesan, C.Umarani, R.Subramanian, N.Nedunchezhian. Bio-diesel as an alternative fuel for
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 245
ISSN 2229-5518
diesel engine- A review. Renewable and Sustainable
Energy Reviews 13 (2009) 653-662.
[19] Rao PS, Gopalakrishnan KV, Use of non-edible vegetable oils as diesel engine fuels, J Inst Eng India
1989; 70(4)
VII.ACKNOWLEDGEMENT
We thank the management of Annamalai University, Annamalai Nagar, Chidambaram- Tamil Nadu for providing us with the necessary experimental set up to perform this research work.
The authors also gratefully acknowledges P.Ramesh Department of Mechanical Engineering for their valuable suggestion on their subjects and also for sharing inputs and important data for this paper.

R.Senthilkumar is a graduate in Mechanical Engineering and post graduate in Thermal Engineering from Annamalai University. Currently he is a research scholar at Annamalai University, Annamalai Nagar. He has over 7 year of teaching experience and has guided
around 8 undergraduate and 3 post graduate theses.
IJSER © 2013 http://www.ijser.org




