Accordingly, the present study is to evaluate the effect of hesperidin on male infertility in streptozotocin induced diabetic rats.
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 2175
ISSN 2229-5518
Pasupuleti Kishore Kumar, Yerraguntla Venu Gopal, Dr. Akondi Butchi Raju
St. Peters Institute of Pharmaceutical Sciences, Hanamkonda, Warangal, Andhra Pradesh, India- 506001.
Dr. Akondi Butchi Raju, Associate Professor, Department of Pharmacology,
St. Peters Institute of Pharmaceutical Sciences, Hanamkonda, Warangal,
Andhra Pradesh, India- 506001. Email ID: drraju2020@gmail.com
Phone: +918008757878
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 2176
ISSN 2229-5518
Effect of hesperidin on diabetes induced infertility
Enhanced oxidative stress and changes in antioxidant capacity are considered to play an important pathogenic role in chronic Type I diabetes mellitus and known to cause infertility in diabetic patients. Plant derived flavonoids like hesperidin are proved to have free radical scavenging capacity. So we studied the effect of hesperidin in type 1 diabetes mellitus induced infertility in male rats. The present study was conducted on 5 groups of male wistar rats (n=6). Treatment group received Hesperidin (HES) 25 and 50 mg/kg (Oral), standard treatment group received insulin 3 IU/kg (Subcutaneous) and diabetic group received streptozotocin (STZ) 40mg/kg (IP), while the normal control group did not receive any drug and were left free with food and water. Animals were kept in standard housing conditions.
On the 40th day the testicular tissue was isolated and the semen samples were collected and
analyzed. Histopathological studies were also conducted. Hesperdin treated groups have shown significant protection and restoration of sperm count, motility, viability. Biochemical parameters like SOD and CAT levels were significantly increased and LPO levels were significantly reduced in treated groups when compared to diabetic group. Histopathological studies showed significant restoration and supported the above claim.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 2177
ISSN 2229-5518
Diabetes Mellitus (DM) type 1 is a chronic disorder which is caused by the failure of insulin production from the Islets of langerhans in Pancreas. It is the most common endocrine disease that leads to metabolic abnormalities involving regulation of carbohydrate metabolism. These abnormalities develop pathologies including vasculopathy, neuropathy, ophthalmopathy, infertility, nephropathy and cardio myopathy (1). Hyperglycemia occurs when the human body tissue cannot store glycogen and utilise glucose. Euglycemic levels can be maintained using oral hypoglycaemic drugs, administration of insulin, treatment of obesity, controlled food intake and regular exercise and yoga (2).
Diabetes Mellitus has been associated with sexual dysfunction, both in men and women. Male infertility is one of the major health problems in life and approximately 90% of diabetic patients suffer from sexual dysfunction that includes reduced libido, ejaculation and impotence (3, 4, 5). Elevated Reactive Oxygen Species (ROS) and alterations in antioxidant defence mechanism play an important pathogenic role in chronic Type I diabetes mellitus. Several observational studies have reported that protection of sperm DNA damage by Reactive Oxygen Species (ROS) by consuming the antioxidants and vitamins like A, B, C & E in the diet can increase the blood flow to the testes and also increase blood testes barrier stability from altering by these free radicals (6, 7).
Increased production of free radicals or ROS formation may induce oxidized Low Density Lipoprotein (Ox-LDL), which is pathogenic step in the sequence of events leading to atherosclosis. Sustained hyperglycemia and increased oxidative stress are the major pathogenic players in the development of secondary complications in diabetes.
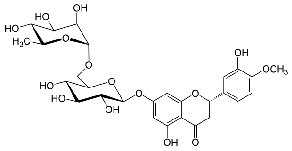
Bioflavonoid compound Quercetin was proved beneficial in diabetes mellitus and also useful in infertility (8). Hesperidin (HPN, 5, 7, 3’-trihydroxy-4’-methoxyflavanone7- rhamnoglucoside) belongs to the class of flavonoids called flavanone is an abundant and inexpensive by-product of Citrus cultivation (9). It exhibits biological and pharmacological properties, such as antiinflammatory, anticarcinogenic, inhibit bone loss, lipid-lowering, hypoglycaemic and antioxidant activities (10, 11). Un Ju Jung and his colleagues have studied hypoglycaemic effects of Hesperidin and Naringin and found that the efficacy is partly mediated by hepatic glucose-regulating enzymes in mice (12). Several studies of researchers have observed antioxidant activity and free radical scavenging properties of
hesperidin using a variety of assay systems (13, 14, 15, 16).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 2178
ISSN 2229-5518

Accordingly, the present study is to evaluate the effect of hesperidin on male infertility in streptozotocin induced diabetic rats.
Streptozotocin was obtained from Himedia laboratories Pvt., Ltd., Mumbai,India Hesperidin was obtained from Spectrochem Pvt., Ltd., Mumbai, India. Insulin was obtained from Torrent pharmaceuticals, Mehsana, India. Glucometer and glucometer strips were obtained from Aspen diagnostics, New Delhi, India.
All experiments were conducted using male wistar albino rats (150-200 g and 6-8 weeks age). All animals were procured from Sainath Agencies, Hyderabad. The animals were maintained with free access to food and water and kept at 25 ± 2◦C under a controlled 12 h light/dark cycle. The care and maintenance of the animals were carried out as per the approved guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi. The research protocols were approved by
the Institutional Animal Ethical Committee (IAEC).
The animals were divided into five groups of six (n=6) animals each. Group I (NC) considered as Normal control, and not administered any drug, and Group II (DC) served as Diabetic control received streptozotocin (40 mg/kg, i.p.), Group III (DI) served as Diabetic control received streptozotocin (40 mg/kg, i.p.)and a standard drug insulin (3 IU/kg, s.c.), Group IV(DIH 25) served as Diabetic control received streptozotocin (40 mg/kg, i.p.) and a standard drug insulin (3 IU/kg, s.c.) and test Hesperidin (25mg/kg, p.o.), Group V (DIH
insulin (3 IU/kg, s.c.) and a test drug Hesperidin (50mg/kg, p.o.).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 2179
ISSN 2229-5518
Rats were kept on fast overnight and injected intraperitonially with STZ, (40 mg / kg b.wt.), freshly prepared in 0.1 M sodium citrate buffer, pH 4.5 (17). During the first 24 hours of diabetes induction, STZ-treated animals were allowed to drink 5% glucose solution to overcome drug-induced hypoglycaemia (18). Treated and control animals were allowed free access to water and standard chow diet. Seventy two hours after STZ administration, diabetes was confirmed by the presence of hyperglycemia and glucosuria. This was found respectively by means of glucometer and glucose strips. STZ-treated animals showed blood glucose more than 300 mg / dL.
Hesperidin was suspended in 0.5% sodium carboxy methyl cellulose and administered at daily oral dose of 25 and 50 mg/kg body weight for a period of 6 weeks. In addition to these diabetic groups, two groups of normal control rats were kept without treatment till the end of the experimental period. Insulin was administered subcutaneously for 40 days alternately for three (3) days each.
The caudal epididymis was dissected out; an incision (about 1 mm) was made in the caudal epididymis. Sperm fluid was then squeezed onto the microscope slide. Epididymal sperm motility was assessed by calculating motile spermatozoa per unit area and was expressed as percent motility. Epididymal sperm counts were made using the hemocytometer and were expressed as million/ml of suspension. The sperm viability was also estimated using Eosin/Nigrosin stains (19).
Testes of the treated rats were taken and fixed in 10% neutral formalin solution. The fixed specimens were then trimmed, washed and dehydrated in ascending grades of alcohol. Specimens were cleared in xylene, embedded in paraffin, sectioned at 4-6 microns thickness and stained with Hematoxylen and Eosin (H & E) dyes, and these were observed microscopically at X 100 (20).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 2180
ISSN 2229-5518
Prior to the antioxidant activity estimations, the animals were sacrificed by decapitation. Testis were removed and rinsed with ice-cold isotonic saline. Testes were then homogenized with ice-cold 0.1 mmol/l phosphate buffer (pH 7.4). The homogenates (10% w/v) were then centrifuged at 10,000 rpm for 15 min and the supernatant so formed was used for the biochemical estimations.
The amount of malondialdehyde (MDA) was measured by reaction with thiobarbituric acid at 532 nm using Spectrophotometer. (21)
Superoxide Dismutase activity in the brain was determined using photo oxidation of o-dianisidine sensitized by riboflavin method of (22). The change in absorbance was recorded for 4 min at 460 nm using Spectrophotometer.
Catalase activity was assessed, based on the ability of catalase to oxidize hydrogen peroxide. The change in absorbance was recorded for 3 min at 1 min interval at 240 nm using Spectrophotometer. (23)
Total protein content was measured by method described in Lowry et al (24). Bovine serum albumin was used as standard.
In the streptozotocin induced Type I diabetic rats sperm count, sperm motility and sperm viability significantly (p<0.05) decreased when compared with normal control group. Treatment with the standard drug (Insulin 3 IU/kg) and Hesperidin (25mg/kg and 50mg/kg), successfully protected normal levels sperm count, sperm motility and sperm viability (p<0.05).Maximum protection was observed when high dose of hesperidin (50 mg/Kg) was
combined with regular dose of insulin. (Table I, Figure 1)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 2181
ISSN 2229-5518
The results showed that lipids of the Type I diabetic rats are vulnerable to peroxidation due to the increased oxidative stress during diabetes (P<0.05). Standard drug insulin and the treatment drug i.e. Hesperidin were all able to reverse the altered LPO damage. The antioxidant enzymes CAT and SOD activities were determined in the testes of diabetic and treated rats and it was compared with control groups. In diabetic treated rats there was a decrease in SOD and CAT activities. The treatment with the standard drug insulin (3 IU/kg) and the treatment drug i.e. Hesperidin (25mg/kg and 50mg/kg) significantly (p<0.05) normalized the altered antioxidant enzymes levels of liver, occurred due to Type I diabetes.(Table II, Figures 2,3,4)
Histopathological examination of testes was done using Hemotoxylene and eosin stain. In diabetic control group there was an inflammation and disturbed testicular architecture when compared with normal control group as shown in the Fig B. Even in insulin treated groups testicular architecture was in disturbed condition, whereas in treated groups (Hesperidin 25mg/kg and 50mg/Kg) testicular architecture largely remained intact. (Figure 5)
Incidence of diabetes mellitus is rising worldwide and will inevitably result in an increased prevalence in men of reproductive age. It is well known that diabetes mellitus has been related with sexual dysfunction. Infertility is already a major health problem in both the developed and developing countries with up to one in six couples requiring specialist investigation or treatment (25, 26). In this aspect, Penson et al. (2009) mentioned that male sexual dysfunction is a common complication of diabetes and the erectile dysfunction, impotence, orgasmic dysfunction, altered ejaculation and decreased libido are highly prevalent in men with chronic diabetes.(27) The ability of a sperm to maintain membrane integrity is critical for survival. A correct approach in exploring sperm oxidative stress in DM patients may start by an initial direct analysis of ROS production in basal and stimulated condition (28).
The patients with diabetes had higher rates of sexual dysfunction than the non- diabetic patients (29). On the other hand, Sandra et al. 2008 reported that diabetes causes
neuropathy and vascular insufficiency that may be associated with sexual dysfunction in men.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 2182
ISSN 2229-5518
(30) Oxidative stress plays a major pathogenic role in the occurrence of secondary complications in diabetes mellitus (8). These complications can be maintained by the use of flavonoids in the diet.
Flavonoids are recommended for their antioxidant properties. The antioxidant property of a flavonoid is determined by its structure and particularly its ability to donate a hydrogen ion to the peroxy radical produced as a result of lipid peroxidation (31,32). In diabetes, testicular dysfunction may be temporary or permanent depending on the degree and duration of the disease. Thus, this disease reduces luteinizing hormone (LH) in serum, which is responsible for normal Leydig cell function (33, 34,35 ). In the present study the diabetic rats have shown drastic reduction in the sperm count and other parameters. Increased oxidative stress and fall in antioxidant reserves was reported. Diabetic rats which treated with Hesperidin have shown protective effect. Almost normal values of sperm parameters, biochemical and histological were observed. The increase in sperm motility of experimental groups in comparison to control group could be due to the protective effect of Hesperidin administration. In the present study, the different doses of Hesperidin were considered, like
25 mg/kg, (p.o) and 50 mg/kg, (p.o.). In this study 25 mg/kg (p.o.) has shown a dose dependent increase in sperm count, sperm motility, sperm viability and data from Histopathological studies confirms this claim. In a recent study, the influence of quercetin (flavonoid) was evaluated on the spermatogenesis of STZ-induced diabetes in male rats.(36) Role of antioxidant supplementation (a mixture of vitamins E and C and α-lipoic acid) on testicular germ cell apoptosis of STZ-induced diabetic rats was already evaluated. (37). our studies on hesperidin suggest greater role of bioflavonoids in the management of male infertility.
The study indicated that streptozotocin induced diabetic mellitus causes reduction in the sperm count, sperm motility, sperm viability and causes histological changes in testis along with the increased oxidative stress. Combination of hesperidin treatment along with insulin succefully protected the testes from the damage and maintained all the parameters at normal level.
1. Sexton WJ, Jarow JP: Effect of diabetes mellitus upon male reproductive function.
J. of Urology. 49, 508 – 513(1997)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 2183
ISSN 2229-5518
2. Rang, H.P, M.M. Dale (1991): Pharmacology. Churchill Livingstone, London, UK.
3. Jiang GY: Practical Diabetes.1st Edition. Beijing: People's Health Publishing
House.295 (1996)
4. Shi-Liang FENG, Shu-Hua LI, Yan WANG, Chang-Chun CHEN, Bin GAO: Effect of ligustrum fruit extract on reproduction in experimental diabetic rats. Asian J. Androl. 3, 71 -73(2001)
5. Isidori, A.M., C.D. Pozza, D. Gianfrilli and A. Isidori: Medical treatment to improve sperm quality. J. Reproduc. Biomed. 12, 704-714 (2006)
6. Baynes JW, Thorpe SR: Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. J. of Diabetes.48, 1 – 9 (1999)
7. Wolff SP, Jiang ZY, Hunt JV : Protein glycation and oxidative stress in diabetes mellitus and ageing. J. Free Radic Biol. Med.10: 339 – 352 (1999)
8. Khaki, A., Nouri, M., Fathiazad, F: Protective Effects of Quercetin on
Spermatogenesis in STZ induced Diabetic Rat. J. Medicinal plants, 8, 57-64 (2009)
9. Garg A, Garg S, Zaneveld LJ, Singla AK Chemistry and pharmacology of the
Citrus bioflavonoid hesperidin. Phytother Res, 15, 655-669 (2001)
10. Bok S.H., S.H. Lee, Y.B. Park, K.H. Bae, K.H. Son, T.S. Jeong and M.S. Choi: Plasma and hepatic cholesterol and hepatic activities of 3- hydroxy-3-methyl- glutaryl-CoA reductase and Acyl CoA: cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. J. Nutr, 129, 1182-1185 (1999)
11. Choi, M.S., K.M. Do, Y.B. Park, S.M. Jeon, T.S. Jeong, Y.K. Lee: Effect of naringin supplementation on cholesterol metabolism and antioxidant status in rats fed high cholesterol with different levels of vitamin E. Ann. Nutr. Metab. 45, 193-
201(2001)
12. Un Ju Jung, Mi-Kyung Lee, Kyu-Shik Jeong, and Myung-Sook Choi: The Hypoglycemic Effects of Hesperidin and Naringin Are Partly Mediated by Hepatic Glucose-Regulating Enzymes. J. American Society for Nutri. Sci., 04, 2499-2503 (2004)
13. Jovanovic SV, Steenken S, Tosic M, Marjanovic B, Simic MG: Flavonoids as anti-
oxidants. J Am Chem Soc. 116, 4846-4851(1994)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 2184
ISSN 2229-5518
14. Fraga CG, Martino VS, Ferraro GE, Coussio JD, Boveris A: Flavonoids as antioxidants evaluated by in vitro and in situ liver chemiluminescence. Biochem Pharmacol. 36, 717-720 (1987)
15. Miller NJ, Rice-Evans CA: The relative contribution of ascorbic acid and phenolic antioxidants to the total antioxidant activity of orange and apple fruit juices and balckcurrant drink. Food Chem, 60, 331-337 (1997)
16. Suarez, Herrera MD, Marhuenda E: In vitro scavenger and antioxidant properites of hesperidin and neohesperidin dihydrochalcone. Phytomedicine,5, 469-473(1998)
17. Wahieb, S.A. and D.V. Godin: Alteration in tissue antioxidant system in the spontaneous diabetic (B B Wester) rats. Cann. J. Physiol. Pharmacol, 65,
2191(1987)
18. Kakkar, R., S.V. Mantha, J. Radhi, K. Prasad and J. Kalra: Antioxidant defense system in diabetic kidney: a time course study, Life Sci. 60, 667-679 (1997)
19. Raji Y, Udoh US, Mewoyeka OO, Onoye FC, Bolarinwa AF: Implication of reproductive endocrine malfunction in male antifertility efficacy of Azadirachta Indica extract in rats, Afr. J. Med. Sci., 32, 159-165 (2003)
20. Luna, L: Manual of Histologic Staining Methods of the Armed Forces Institute of
Pathology. McGraw-Hill Book Company, New York, pp. 65–88. (1968)
21. Okhawa H, Ohishi N, Yagi K: Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Ann Biochem, 95, 351–358 (1979)
22. Arutla S, Arra G. S, Prabhakar C. M, Krishna D.R: Pro- and Anti-Oxidant Effects of Some Antileprotic Drugs in Vitro and Their influence On Super Oxide Dismutase Activity. Arzneim.-Forsch, J Drug Res.,48, 1024 (1998)
23. Beer RF, Seizer TW: A spectrophotometric method for measuring breakdown of hydrogen peroxide by catalase. J Biol Chem., 115, 130–140 (1952)
24. Lowry, O.H: Colorimetric assays: Lowry method for protein determination, J. Biol.
Chem. 193, 265-275 (1951)
25. Hull MG, Glazener CM, Kelly NJ: Population study of causes, treatment, and outcome of infertility. Brit Med J (Clin Res Ed),291, 1693–1697 (1985)
26. Schmidt L, Munster K : Infertility, involuntary infecundity, and the seeking of medical advice in industrialized countries: a review of concepts, measurements and
results. Hum Reprod., 10, 1407–1418 (1992)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 2185
ISSN 2229-5518
27. Penson, D.F., Wessells, H., Cleary, R., Rutledge, B.N: Sexual dysfunction and symptom impact in men with long standing type 1 diabetes. Journal of Sexual Medicine, 6, 1969–1978(2009)
28. S. La Vignera, E. Vicari, A.E. Calogero, R. Condorelli, F. Lanzafame: Diabetes, oxidative stress and its impact on male Fertility J. of Andro. Sci., 16, 42-46(2009)
29. Ziaei-Rad, M., Vahdaninia, M., Montazeria, A: Sexual dysfunction in patients with diabetes: a study from Iran. Reproductive Biology and Endocrinology, 8, 50(2010).
30. Sandra, A., Paulo, J.O. and Joao, R: Diabetes and the impairment of reproductive functions: Possible role of mitochondria and reactive oxygen species. Current Diabetes Reviews, 4, 46-54 (2008)
31. Kashima, M: Effects of catechins on superoxide and hydroxyl radical. Chem.
Pharm. Bull, 47, 279–283(1999)
32. Saija, A., Scalese, M., Lanza, M., Marzullo, D., Bonina, F.,Castelli : Flavonoids as antioxidant agents: importance of their interaction with biomembranes. Free Radic. Biol. Med., 19, 481–486(1995)
33. Custro N, Carroccio A, Ganci A, Scafidi V, Campagna P, Di Prima L: Glycemic homeostasis in chronic viral hepatitis and liver cirrhosis. Diabetes Metab, 27, 476
– 481(2001)
34. Lecube A, Hernandez C, Genesca J, Esteban JI, Jardi R, Simo R: High prevalence of glucose abnormalities in patients with hepatitis C virus infection: a multivariate analysis Considering the liver injury. Diabetes Care, 27, 1171 – 1175 (2004)
35. Skibola CF, Smith MT: Potential health impacts of excessive flavonoid intake. J.
Free Radic Biol. Med., 29: 375 – 383(2000).
36. Khaki, A., F. Fathi Azad, H.R. Ahmadi Ashtiani, S.H. Rezazadeh, H. Rastegar and A.M. Imani: Compartments of quercetin and Allium cepa (onion) on blood glucose in diabetic rats. J. Med. Plans, 9, 107-112 (2010)
37. Sandro La Vignera, Rosita Condorelli, Enzo Vicari, Rosario D’Agata, Aldo E.
Calogero: diabetes mellitus and sperm parameters: a brief review. J. Andro,25, 01-
19(2011).
IJSER © 2013 http://www.ijser.org