4 is a selective PPARγ partial agonist (Figure 2 and 3). These results suggest that PPAR-4 acts as a specific PPARγ partial agonist.
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 627
ISSN 2229-5518
Dwivedee Mithilesh*; Ahuja Anil; Chaudhary Sumit; Dube Aakanksha
Abstract— The peroxisome proliferator activated receptors (PPARs) are nuclear receptors that play key roles in the regulation of lipid metabolism and differentiation. Herewith characterized the pharmacological profiles of PPAR-4 chemically known as (5Z)-5-[3, 4, 5 trimethoxy-phenyl) methylene] thiazolidine-2, 4-dione), as a selective PPARγ agonist. In transient transactivation assay in NIH3T3 cells, PPAR-4 showed a activation against human PPARγ with an EC 50 of 1.01 μM without activating human PPARα and PPARδ. In adipocyte differentiation assay, PPAR-4 induced adipocyte differentiation, which was ~30-fold weaker inducer of GPDH activities than pioglitazone and also showed weak adipogenic activity in C3H10T1/2 pluripotent stem cells using Oil Red O staining. These results suggest that PPAR-
4 acts as a selective partial PPARγ agonist having weak adipogenic activity.
Key words: Peroxisome proliferator-activated receptors (PPARs), Insulin resistant, adipocyte differentiation, adipose tissue.
—————————— ——————————
Peroxisome proliferator activated receptors (PPARs) are ligand activated transcription factors belonging to the nuclear receptor superfamily.1 Three distinct receptor subtypes, PPARα, PPARγ and PPARδ(δ), have been identified and cloned. While the PPAR subtypes share a high level of sequence and structural homology, each has distinct physio- logical functions and each PPAR subtype exhibits a unique tissue expression pattern. PPARα is found in tissues with high rates of fatty acid catabolism and is highly expressed in brown adipose tissue, followed by liver, kidney, heart, and skeletal muscle.2 This receptor regulates genes that control reverse cho- lesterol transport as well as the transport and degradation of free fatty acids through peroxisomal and beta-oxidation path-
ways.
PPARγ, the most widely investigated PPAR subtype, is ex-
pressed predominately in adipose tissue with lower levels expressed in heart, colon, kidney, spleen, intestine, skeletal muscle, kidney, liver and macrophages.2 PPARγ is widely rec- ognized as a pivotal transcription factor in the regulation of adipocyte gene expression and differentiation. The participa- tion of PPARγ in adipocyte differentiation involves a complex coordinated signaling cascade with other families of transcrip- tion factors.3 In addition; PPARγ has been shown to be an im- portant regulator of target genes involved in glucose and lipid metabolism.
Dr. Anil Ahuja. Department of Pharmacology, Faculty of pharmacy, Rajasthan college of pharmacy, Jaipur, Rajasthan, India
Mithilesh Dwivedee. Department of Pharmacology, Faculty of pharmacy, Varanasi college of pharmacy Babatpur, Varanasi, Uttar Pradesh, India
The PPARs modulate the expression of numerous target genes that play a central role in regulating glucose, lipid and choles- terol metabolism where imbalances can lead to diabetes, obesi- ty and cardiovascular disease.4,5
PPARγ receptor agonists display differential physical interac-
tion with the receptor and are classified as either full or partial agonists based on maximal efficacy that they exhibit in cell based transactivation assays. Recent studies have indicated that partial PPARγ agonist’s exhibit improved safety margins compared to full PPARγ agonists and consequently much ef- fort has been put in promoting these partial PPARγ agonists for clinical development. 6
These advantages have initiated the search for some novel
potential partial PPARγ agonist with lesser side effect. In an effort to search for novel PPARγ agonists, we screened a li- brary of various structurally diverse synthetic compounds. Among active compounds identified a compound with indene structure was chosen based on the novelty and ease of deriva- tives synthesis and chemical modification of this molecule lead to the PPAR-4 as a lead compound for novel partial PPARγ agonists.
PPAR-4 and pioglitazone were synthesized at Poona College of pharmacy, Pune, India. The compounds were dissolved in dimethyl sulfoxide (DMSO) and added to medium to a final DMSO concentration of 0.1% for in vitro studies.
mithu_cology@yahoo.co.in
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 628
ISSN 2229-5518
The ligand binding domains of hPPARα (amino acids 167–
468), hPPARδ (amino acids 167–441) and hPPARγ (amino ac- ids 163–477) were generated by PCR amplification using Pfu polymerase and gene specific primers flanked with restriction enzymes BamHI and XbaI. The ligand binding domains were subcloned in-frame into the Pfacmv vector (Stratagene) to prepare pFA-Gal4-PPARα-LBD, -PPARδ- LBD and -PPARγ- LBD. At 75–90% confluence, NIH3T3 cells were transiently co- transfected with one of the expression vectors for pFAGal4- PPAR-ligand binding domains together with pFR-Luc and pRLCMV (Promega) using Lipofectamine plus reagent. Fol- lowing 24 h incubation, the cells were treated with various concentrations of PPAR-4 and incubated for 16 h. Luciferase assay was performed using dualluciferase reporter assay sys- tem and the activity was determined in Microlumat plus Lu- minometer by measuring light emission for 10 s. The results were normalized to the activity of renilla expressed by co- transfected Rluc gene under the control of a constitutive pro- moter.7
NIH3T3L1 mouse fibroblasts (American Type Culture Collec- tion, Rockville, MD) were maintained in Dulbecco'smodified Eagle'smedium (DMEM) supplemented with 10% fetal calf serum (FCS) and 1 mg/l gentamicin. The cells were seeded and grown to confluence in a 24-well culture plate in a 5% CO2 atmosphere at 37 °C. At postconfluence, the mediumwas changed to DMEM/5% FCS supplementedwith 0.5 mmol/l 1- methyl-3-isobutylxanthin, 0.5 μmol/l dexamethasone, and 1 mg/l gentamicin for 48 h. The medium was then treated with test chemicals in DMEM/5% FCS (23). The activities of glycer- ol-3-phosphate dehydrogenase (GPDH) in the cells were measured at 9 days postconfluence as described.8
The adipogenic potency of PPAR-4 was determined as de- scribed previously.9 Briefly, C3H10T1/2 pluripotent stem cells were grown in DMEM supplemented with 10% fetal calf se- rum. Confluent cells were incubated with various concentra- tions of PPAR-4 or pioglitazone in the presence of insulin (200 nM) with medium change every 2–3 days. After 7–9 days of differentiation, the cells were fixed and stained with Oil Red O for 1 h. Oil Red O was prepared by diluting a stock solution (0.5 g/10 ml isopropanol) with water (6:4).
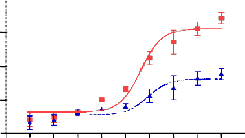
GAL4- responsive reporter gene plasmid, PPAR-4 induced a transactivation activity in a concentration-dependent manner with a maximum activation equal to 50% of Pioglitazone (up to 50-fold), indicating that PPAR-4 was a partial agonist (Fig- ure 1). An EC50 was estimated to be 1.01 μM while that of Pioglitazone was 0.5 μM. PPAR-4 was unable to activate PPARα and PPARδ in a cell based assay, indicating that PPAR-
4 is a selective PPARγ partial agonist (Figure 2 and 3). These results suggest that PPAR-4 acts as a specific PPARγ partial agonist.
100
75
50
25
0
-12 -11 -10 -9 -8 -7 -6 -5 -4 -3 -2
Conc. (Log M)
![]() Pioglitazone
Pioglitazone
![]()
PPAR-4
7
6
5
4
3
2
1
0
![]()
PPAR-4 (1 µM ) PPAR-4 (3 µM ) PPAR-4 (10 µM ) PPAR-4 (30 µM )
![]() Piogliatazone (30 µM)
Piogliatazone (30 µM)
![]() WY14643 (30 µM )
WY14643 (30 µM )
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 629
ISSN 2229-5518
![]()
![]() GW 501516 (30 nM)
GW 501516 (30 nM)
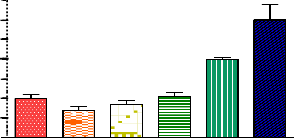
Various groups have reported that PPARγ ligands promote adipocyte differentiation in various cultured fibroblasts and mesenchymal stem cell line systems.10 Thus, we next examined whether PPAR-4 promotes the terminal differentiation of the preadipocyte cell line NIH3T3L1. As markers of adipocyte differentiation, we measured increases in the activities of GPDH in cells treated with various concentrations of PPAR-4 or pioglitazone as positive control thiozolidinedione (Figure
4). PPAR-4 promoted differentiation in a dose-dependent
manner, although it was less effective than the pioglitazone. PPAR-4 was a ~30-fold weaker inducer of GPDH activities than pioglitazone. The concentrations of various thiozoli- dinedione required for adipocyte differentiation of cultured fibroblasts differed significantly, but these differences did not directly correspond to their antidiabetic activities.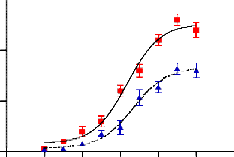
![]()
![]()
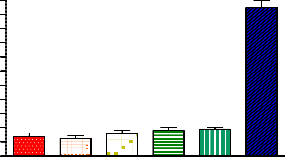
It has been shown that PPARγ agonists induce adipogenesis of a variety of preadipocytes and stem cell lines into mature adi- pocytes, indicating that PPARγ plays a key role in adipocyte differentiation. Using C3H10T1/2 pluripotent stem cells, we found that PPAR-4 showed weak adipogenic activity, as indi- cated by the lack of the adipocyte phenotype, and little Oil Red O staining (Figure 5).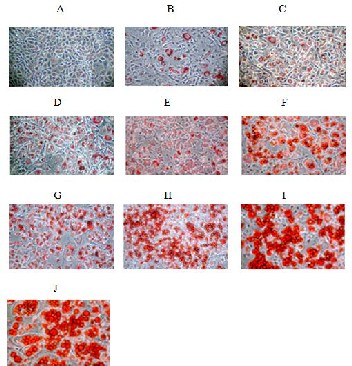
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 630
ISSN 2229-5518
A= Control
B= Insulin alone
C= Insulin + PPAR-4 (0.1 μM ) D= Insulin + PPAR-4 (1 μM ) E= Insulin + PPAR-4 (10 μM ) F= Insulin + PPAR-4 (100 μM )
G= Insulin + Pioglitazone (0.1 μM )
H= Insulin + Pioglitazone (1 μM ) I= Insulin + Pioglitazone (10 μM ) J= Insulin + Pioglitazone (100 μM )
PPARγ agonists (e.g., rosiglitazone and pioglitazone) are widely used as oral anti-diabetic agents by increasing insulin sensitivity and improving glycemic control in type 2 diabetes. However, these compounds induce adipogenesis in cell cul- ture models and increase weight gain in rodents and hu- mans.11
Due to the undesired side effects of thiazolidinediones includ-
ing weight gain, novel PPARγ modulators that retain effica-
cious insulin sensitizing action while minimizing potential side effects are in need. The current study describes the activi- ty profiles of a novel Partial PPARγ agonist, PPAR-4 with the aim of the discovery of safe and efficacious anti-diabetic agents. PPAR-4 exhibited unique pharmacological activities having glucose lowering activity with less adipogenesis in vitro.
Results from our in vitro studies showed that PPAR-4 has a unique profile compared with the well-characterized PPARγ agonist pioglitazone. PPAR-4 binds to PPARγ with low affini- ty and no affinity towards PPARα and PPARγ. PPAR-4 also showed less adipogenesis and adipocyte differentiation as compare to full PPARγ agonist pioglitazone. The PPARγ par- tial agonist activity of PPAR-4 may become a distinct ad- vantage for this compound because a number of studies have shown that PPARγ partial agonists including selective PPAR modulators have improved side effect profiles compared with full agonists.12,13,14
Moreover, PPARγ is the most predominant adipogenic recep-
tor and preferentially binds to two regulatory sequences de-
rived from a fat specific gene, which suggests that the abun- dant adipogenic potential of PPARγ may be conferred by its ability to bind to fat specific DNA regulatory sequences.15 To address the possibility that PPAR-4 regulates adipocyte differ- entiation, we assessed the adipogenic potential of PPAR-4 us- ing preadipocyte cell line NIH3T3L1. In these cells, PPAR-4 induced adipocyte differentiation which means that the
PPARγ component of PPAR-4 contributes at molecular level to adipocyte differentiation. However, this potency of PPAR-4 was relatively lower than that of pioglitazone. Similarly in adipogenesis assay using C3H10T1/2 pluripotent stem cells, PPAR-4 was found to be approximately 30 folds inferior to Pioglitazone.
Taken together, the results of the present study demonstrate
that PPAR-4: (1) is functionally active as a selective partial PPARγ agonist shown by transactivation assay, (2) is little ad- ipogenic, thus blocking Pioglitazone induced conversion of preadipocytes to adipocytes.
In conclusion, PPAR-4 is a novel and selective PPARγ modula- tor, with different activity profiles from Pioglitazone. PPAR-4 with modified and selective pharmacological profiles may offer benefit for the treatment of type 2 diabetes and obesity. Further optimization of PPAR-4 and detailed side effect pro- files reported for conventional PPARγ agonists are in progress.
1. Berger, J., Moller, D.E., 2002. The mechanisms of ac- tion of PPARs. Annu Rev Med. 53, 409–435.
2. Escher, P., 2001. Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeed- ing. Endocrinology. 142 (10), 4195–4202.
3. Spiegelman, B.M., Flier, J.S., 1996. Adipogenesis and obesity: rounding out the big picture. Cell. 87 (3), 377–
389.
4. Balint, B.L., Nagy, L., 2006. Selective modulators of PPAR activity as new therapeutic tools in metabolic diseases. Endocr Metab Immune Disord Drug Tar- gets. 6 (1), 33–43.
5. Haluzik, M.M., Haluzik, M., 2006. PPAR-alpha and
insulin sensitivity. Physiol Res. 55 (2), 115–122.
6. Lalloyer, F., Staels, B., 2010. Fibrates, glitazones, and
peroxisome proliferator-activated tors. Arterioscler Thromb Vasc Biol. 30, 894–899.
7. Kwang, R.K., Jeong, H.L., Seung, J.K., Sang, D.R., Won, H.J., Sung, D.Y., 2006. a novel peroxisome pro- liferator-activated receptor γ agonist with weak adi- pogenic effects. Biochem Pharmacol. KR-62980, 72,
446–454.
8. Wise, L.S., Green, H., 1979. Participation of one iso-
zyme of cytosolic glycerophosphate dehydrogenase in
the adipose conversion. J Biol Chem. 254, 273–275.
9. Sen, A., Lea-Currie, Y.R., Sujkowska, D., Franklin,
D.M., Wilkison, W.O., Halvorsen, Y.D., 2001. Adipo-
genic potential of human adipose derived stromal
cells from multiple donors is heterogeneous. J Cell Bi-
ochem. 81, 312–319.
10. Yuka, F., Sei-ichiro, M., Shiho, O., Kazuhiko, U., Kiyo-
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 631
ISSN 2229-5518
to, M., 2000. A new thiazolidinedione, NC-2100, which is a weak PPAR-γ activator, exhibits potent an- tidiabetic effects and induces uncoupling protein 1 in white adipose tissue of KKAy obesemice. Diabetes.
49, 757–767.
11. Schoonjans, K., Staels, B., Auwerx, J., 1996. The perox-
isome proliferator activated receptors (PPARs) and
their effects on lipid metabolism and adipocyte differ-
entiation. Biochim Biophys Acta. 1302, 93–109.
12. Berger, J.P., Petro, A.E., Macnaul, K.L., Kelly, L.J.,
Zhang, B.B., Richards, K., 2003. Distinct properties
and advantages of a novel peroxisome proliferator-
activated protein γ selective modulator. Mol Endo-
crinol. 17, 662–676.
13. Rocchi, S., Picard, F., Vamecq, J., Gelman, L., Potier,
N., Zeyer, D., 2001. A unique PPAR γ ligand with po-
tent insulin-sensitizing yet weak adipogenic activity.
Mol Cell. 8, 737–747.
14. Shimaya, A., Kurosaki, E., Nakano, R., Hirayama, R.,
Shibasaki, M., Shikama, H., 2000. The novel hypogly-
cemic agent YM440 normalizes hyperglycemia with-
out changing body fat weight in diabetic db/db mice.
Metabolism. 49, 411–417.
15. Brun, R.P., Tontonoz, P., Forman, B.M., Ellis, R.,
Chen, J., Evans, R.M., 1996. Differential activation of
adipogenesis by multiple PPAR isoforms. Genes Dev.
10, 974–984.
IJSER © 2014 http://www.ijser.org