International Journal of Scientific & Engineering Research, Volume 5, Issue 5, May-2014 117
ISSN 2229-5518
Oxidative Stress markers, 8-isoprostane &
advanced oxidation protein products (AOPPs), in Acute Myocardial Infarction patients with
acute Hyperglycemia
Nahla S. Hassan*, Nevien A. Mahran, Shireen F. Tawfik, Ibrahim H. Borai
63 years with no known history of MI were admitted to hospital within 12 h of MI onset. MI diagnosis was established by four criteria: (1) chest pain of >30 min; (2) ST segment-alteration; (3) serum creatinine kinase-MB (CK-MB) > 25 U/L; and (4) troponin I levels of >
0.3 ng/mL. All patients were positive for all four criteria. Serum CK activity and TnI levels were measured on the day of hospital
admission. Twenty healthy subjects matched in age and sex was selected as control. Plasma or sera of all subjected were quantitatively analyzed for lipid profile, 8-isoprostane, AOPPs, t-Hcy and serum CRP. Results: Our results indicated that both 8- isoprostane and AOPPs are early, highly sensitive and specific markers for the diagnosis of AMI with acute hyperglycemia . The study also confirmed the diagnostic value of sCRP and t-Hcy. NEFA yielded a significantly worse accuracy for diagnosing AMI.
—————————— ——————————
the topic of triggers and their possible prevention
(Colombo et al., 2014).
Hyperglycemia and hypoglycemia are associated with
cardiovascular events in patients with coronary artery
disease (CAD), regardless of their diabetic status. The relationship between glucose levels and increased mortality risk in cases of acute myocardial infarction (MI) has been established through various glucose metrics (Madani et al., 2013).
The role of oxidative stress in ischaemic heart disease has been thoroughly investigated in humans. Increased levels of ROS (reactive oxygen species) and RNS (reactive nitrogen species) have been demonstrated during ischaemia and post-ischaemic reperfusion in humans. Depending on their concentrations, these reactive species can act either as benevolent molecules that promote cell survival (at low-to-moderate concentrations) or can induce irreversible cellular damage and death (at high concentrations) ( RODRIGO et al., 2014).
Oxidative stress is usually associated with increased
formation of reactive oxygen species (ROS) that play central roles in cardiac physiology and pathophysiology. It appears that hyperglycemia per se can induce oxidative stress. In fact, a feature common to all cell types that are damaged by hyperglycemia is an increased production of ROS. Particularly, increased production of mitochondrial ROS by hyperglycemia is recognized as a major cause of the clinical complications associated with diabetes. An overproduction of superoxide by the mitochondrial electron transport during hyperglycemia has been documented (Di Filippo et al., 2006). Here, we report some important oxidative stress and inflammatory markers in AMI patients with acute hyperglycemia.
Coronary heart disease (CHD) has been the leading cause of death for the last 10 years, as announced by the
World Health Organization, (Wang and Ge, 2014) accounting for 11.24% of all deaths in 2011. Its primary and secondary prevention is of great importance.
Acute myocardial infarction (AMI) defines a sudden blockage of coronary arteries with myocardial ischemia, injury and necrosis. The major clinical manifestations are
intense pectoralgia felt behind the breast bone, special dynamic changes of the electrocardiogram and of myocardial enzymes. The incidence is increasing and it is life-threatening if not treated (Sangu et al., 2012). The infarction-related artery (IRA) accessed within 12 hours by direct percutaneous coronary intervention (PCI), is the first treatment option for patients with acute ST-segment elevation myocardial infarction (STEMI), Levine et al., (2011). The treatment can improve hemodynamic, reduce complications, and improve prognosis (Shen, 2012 & Yanfei et al., 2014).
The existence of specific risk factors for the
development of coronary heart disease, both chronic
and acute, has been extensively investigated and is well
understood by cardiology professionals. Diabetes,
hypertension, hypercholesterolemia, psychological patterns and smoking are assumed to interact in a complex way with individual heritable predisposition, thus determining the long-term probability of coronary disease. However, the possibility that defined circumstances and activities may act as immediate triggers of acute coronary syndromes, particularly acute myocardial infarction, has not been given comparable attention in clinical research. For example, the recently issued 2012 European guidelines on cardiovascular disease prevention completely overlook
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 5, May-2014 118
ISSN 2229-5518
57.26% respectively (P<0.001) when compared to the control group. Meanwhile, Plasma GSHPx levels were decreased significantly in patient groups (P<0.001) by -
63.67%, -66.90%, respectively when compared with control group. Beside considered a marker of inflammation, CRP is now considered a biomarker of several cardiac conditions. It was significantly increased by 26.29% in group II (P<0.05) and 167.61% in group III (P<0.001) when compared to the control group.
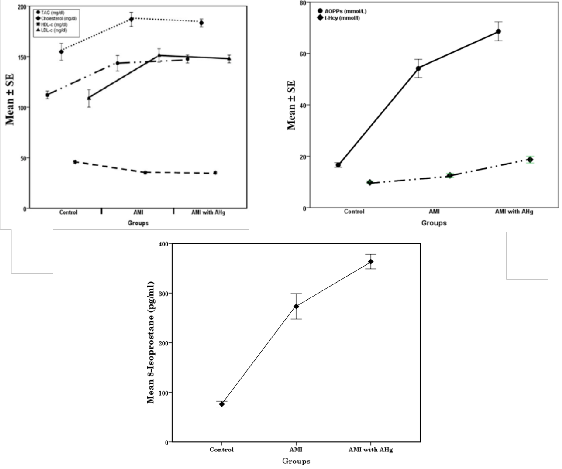
Data in figure 1 showed that, lipid profile (figure 1a)
revealed that there was a significant increase in the level
of TG, TC and LDL-c in groups II and III compared to the
normal control group, while a significant decrease in
HDL-c was observed in the two studied groups
compared to the normal control group. Plasma levels of advanced oxidation protein products (AOPPs) and t-Hcy were highly significantly increased (P<0.001) in patient groups except t-Hcy was significantly elevated (P<0.05) in group II. Moreover, F8-Isoprostane was increased by
259.03% (G II) and 377.66% (G III) as indicated in figure
(1b & c).
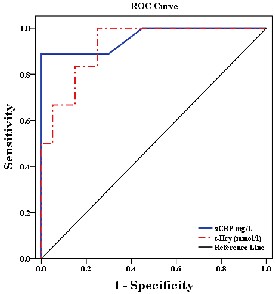
The Receiver Operating Characteristic (ROC) curve
and areas under the curves (AUC) for 8-Isoprostane &
AOPPs are presented in figure and table 2. NEFA yielded
a significantly worse accuracy for diagnosing AMI, while, LDL-c showed highest diagnostic performance. The level of detection as cut-off and different tested relative changes are provided in (Table 3 & Fig.3).
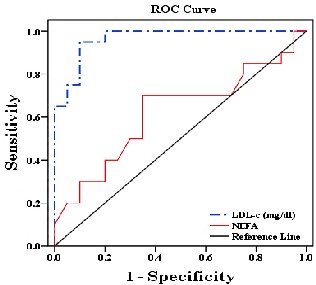
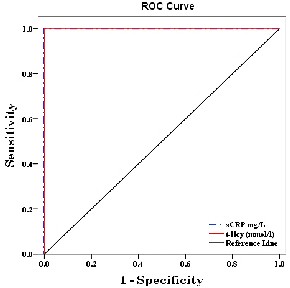
The sCRP & t-Hcy provided the highest diagnostic information of these biomarkers, with an AUC and cut off
values are shown in table and Fig. 4.
Management of acute myocardial infarction and its
complications represent decisive challenges in contemporary cardiology. Prevention, early diagnosis, and prompt management are essential to improve outcome and decrease complications.
Fundamental mechanisms such as endothelial dys-
function, oxidative stress, and inflammation are involved in the pathophysiology of most acute cardiovascular conditions (Kossaify et al., 2013).
Cardiac BMs (CBs) are generally the degradation product of myocardial cells, metabolites, hormones, enzymes, or simple serum markers, such as creatinine.
CBs reflect different pathological processes including cardiac injury and necrosis, myocardial stress, inflam- mation, and plaque destabilization (Singh et al., 2012). First described in 1965, creatine kinase (CK) was the first CB used to assess myocardial infarction. CK myocardial band (CK-MB), a more specific indicator, followed in
1972. In 1989, the next major advance in CB development
was the introduction of cardiac troponin (cTn) (McLean
and Huang, 2012). CBs provide insights into variable
physiopathological features such as oxidative stress,
inflammation, platelet activation, and neurohormonal activity (Loria et al., 2008). In view of this, assessment via multi-markers assays may help to adjust treatment according to the underlying physiopathological mechanism (Aldous, 2013).
This study included 38 male patients, selected from those attending the Intensive Care Unit (ICU) of Al- Hussein hospital, Al-Azhar University, aged 40 - 63 years (mean age of 53±9.65 years) with no known history of MI admitted to hospital within 12 h of MI onset. Serum CK activity and TnI levels were measured on the day of hospital admission. Twenty healthy subjects matched in age and sex was selected as control. A full medical history was taken with special attention to any associated medical problems. The exclusion criteria for patients and controls were: muscle injury, muscle disease, intense exercise, renal impairment, liver disease, bronchial asthma, diabetes insipidus, symptoms or signs of heart valve disease, cardiomyopathy, endocrinal or metabolic disorders and cancer.
Subjects in this study were classified into three
groups: Group I, Include 20 apparently healthy control
subjects, with no history of heart diseases and normal
electrocardiographic findings, Group II, Include 18 acute
myocardial infarction (AMI) patients and Group III, Include 20 AMI patients with acute hyperglycemia (AHg). 2 ml of blood was collected on EDTA coated tube for HbA1c according to Trivelli (1971). Separated plasma was used for measuring glutathione peroxidase (GSHPx) according to Paglia and Valentine (1967) & AOPPs (Witko-Sarsat et al., 1996). The rest of blood sample was kept in clean glass tube without additives to clot at 37 ºC for 20 minutes, and then centrifuged at 3000 rpm for 10 minutes. The sera of all studied groups were subjected to the following investigations, total lactate dehydrogenase (LDH) as described by Everse and Kaplan (1973), cardiac Troponin I (c-TnI) according to Takahashi et al., (1996), total creatine kinase (t-CK) was carried out according to the method of Gruber, (1978). Creatine kinase isoenzyme MB (CK-MB) was determined by the method of Meiattini, (1978), glucose (Trinder, 1969), total cholesterol (Richmond, 1973), triacylglycerol (Young and Pestaner,
1975), (LDL-c) (Freidwald et al., 1972), (HDL-c) (Lopes-
Virella 1977) and non-esterified fatty acids (NEFA) by
Henry (1974). Moreover, serum C-reactive by Roberts,
(2000) & nitric oxide via measuring nitrite (NO2) by Miranda et al., (2001) were carried out. 8-isoprostane was determined by enzyme-linked immunesorbant assay (ELISA) (Morrow, 2000).Total homocysteine (t-Hcy) was determined by the method of Refsum et al., (1998). Statistical analysis was carried out by the aid of a digital computer, using Excel, and IBM SPSS Statistics version 21 program.
Our results in table 1 indicated that, fasting blood
glucose was highly significant elevated (p<0.001) in the studied patients groups II & III by 86.69% and 247.04% respectively when compared with control group while, HbA1c was highly significantly increased (p<0.001) in group III by109.08% & non-significantly changed in group II. In the meantime, common cardiac biomarkers (LDH, CK, CK-MB and T nI) were highly significant increased (p<0.001) when compared with control group I.
Nitric oxide (NO) was highly significantly decreased in the two patient groups (G II&III) by -38.39% and -
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 5, May-2014 119
ISSN 2229-5518
HbA1c was significantly elevated in group III compared to the other two groups which is due to the level of the sugar.
C-reactive protein is an acute phase reactant marker for underlying systemic inflammation CRP has been reported to be elevated in MI patients. In this study, we observed increased CRP levels in all studied groups .The highest concentration was observed in the AMI with hyperglycaemia. Our results are in agreement with Kasap et al., (2007), who reported that patients with AMI had significantly higher CRP levels than control group. The author also indicated that diabetic AMI patients also had CRP levels higher than control. Fujii et al., (2002) also reported significant increase in CRP level in case of AMI.
Kasap et al., (2007) reported that in MI patients, and also in diabetic MI patients, the total cholesterol and LDL- cholesterol were higher but HDL- cholesterol was lower than the healthy control and there were no differences in triglycerides and VLDL-cholesterol.
Lv et al., (2013) showed significant differences in TC, HDL and LDL between AMI patients and control group. The levels of TC and LDL were significantly elevated while the level of HDL was significantly decreased. However non significant increase was found in the level of TG.
Our findings indicated non –significant change in the
lipid profile (TC, TG, HDL-c and LDL-c) between
hyperglycaemic AMI patients and AMI patients, these
results are in consistent with Ekmekci et al., (2013).
Havmoeller et al., (2014) findings strengthen the role
of plasma NEFA as a potential biomarker for the assessment of sudden cardiac death risk, where our results revealed non- significant increase in the NEFA in the studied groups.
Oxidative stress plays a major role in the pathogenesis
of heart failure, and previous studies showed that prolonged increased oxidative stress was related to an impaired prognosis in heart failure patients ( Cai and Harrison, 2000; Crisby et al., 2009).
Isoprostanes also appear to be reliable markers of
ischemic tissue injury. For example, increased levels of F2-isoprostanes have been noted after ischemia/reperfusion induced by percutaneous coronary intervention. Furthermore, Pericardial levels of F2- isoprostanes increase with the functional severity of congestive heart failure and are associated with pathologic cardiac remodeling. Plasma levels of F2- isoprostanes are also increased in patients with chronic heart failure in relation to disease severity and the degree of cardiac dysfunction ( Polidori et al., 2004) .This is confirmed by our results, which showed significant elevation in the level of 8-isoprostane in all the patient groups compared to the healthy volunteers.
AOPP is formed during oxidative stress by the
reaction of plasma protein with chlorinated oxidants, and
it was suggested as a measure of highly oxidized
proteins, especially albumin.
This study indicated a significant increase in the
AOPP level in all the patient groups compared to the
healthy control group. Recently, evidence has been
provided that AOPP are pro-inflammatory mediators that
directly impair HDL metabolism and might therefore be
Our Results indicated a significant increase in LDH, CK, CK-MB and TnI in the two patients groups compared to the healthy control group.
Gopcevic et al., 2011 also reported significant increase in the serum LDH in AMI patients. The elevation of LDH is attributed to the intense anaerobic respiration in AMI, so there is increased lactate production and consequently higher LDH activity in sera.
CK and more particularly its isoenzyme CK-MB still have a formal place in defining myocardial infarction. In this study, as expected, CK and CK-MB levels in patients with AMI were higher than healthy control. We have found a significant elevation in their levels in group of hyperglycemia compared to AMI group. These results are in consistent with Kasap et al., (2007).
Some Patients frequently develop elevated blood
sugars as a response to stress. Stress induced
hyperglycemia (SIH) refers to a complex metabolic
response to stress through raised catecholamine and
stress hormones resulting in elevated blood sugar levels (Lionel et al.,2014). Our results indicated a significant elevation in the level of glucose in the patients groups compared to the healthy volunteers.
Hyperglycemia during acute myocardial infarction
(AMI) is associated with a poor prognosis, and blood glucose level is an independent predictor of mortality in patients with or without known diabetes. The future glycometabolic profile of patients suffering AMI without diabetes can be predicted in the hospital phase. There is also a correlation between blood glucose on hospital admission for AMI and long-term mortality in patients with or without known diabetes (Cakmak et al., 2008).
Elevated glucose levels, in our studied groups, have detrimental effects via several mechanisms. Glucose can induce reactive oxygen species generation through the
activation and induction of NADPH oxidase-based mechanisms in healthy subjects (Mohanty et al., 2000). In hyperglycaemic patients with acute myocardial infarction, this increased oxidative stress, combined with increased inflammation and apoptosis, results in a lower left ventricular ejection fraction (Dandona et al., 2007). Secondly, hyperglycaemia influences coagulation, as it is associated with increased platelet aggregation, circulating clotting factors and tissue factor (Dandona et al., 2005). The latter is an activator of thrombotic mechanisms and matrix metalloproteinases, which destabilize the atherosclerotic plaque and mediate its rupture. Another mechanism is the no-reflow phenomenon, reflecting microvascular dysfunction, which is more common in hyperglycemic patients (Iwakura, 2003). This dysfunction is partly explained by impaired perfusion as a result of endothelial dysfunction (Dandona et al., 2005).
Glycated hemoglobin A (HbA1c) expressed as a
percentage of adult hemoglobin that is glycated is the
most widely used measure of chronic glycemia and
provides intensive care physicians a means to detect
those with diabetes and differentiate them from those with Stress induced hyperglycemia (Lionel et al.,2014). Glycosylated hemoglobin (HbA1c) level on admission is a prognostic factor for mortality in patients with and without diabetes after myocardial infarction (Cakmak et al., 2008).
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 5, May-2014 120
ISSN 2229-5518
The authors would like to acknowledge Prof. Dr. Fahmy T. Ali, professor of Biochemistry for his continuous assistance and valuable suggestions in this work.
[1] Wang Z and Ge J. 2014. Managing hypercholesterolemia and preventing
cardiovascular events in elderly and younger Chinese adults: focus on rosuvastatin. Clinical Interventions in Aging ; (9): 1–8.
[2] Sangu PV, Ranasinghe I, Aliprandi Costa B, Devlin
G, Elliot J, Lefkovitz J, et al. 2012.Trends and
predictors of rehospitalisation following an acute coronary syndrome: report from the Australian and New Zealand population of the Global Registry of Acute Coronary Events (GRACE). Heart; (98): 1728- 1731.
[3] Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation; (124): e574-e651.
[4] Shen WF. 2012. Direct intracoronary delivery of tirofiban during primary percutaneous coronary intervention for ST-elevation myocardial
infarction. Chin Med J; (125): 3-6.
[5] Yanfei W., Min Y., Haibo L., Yuejin Y., Junmin X.,
Xinwei J., Huanjun P. and Chunyan W.
2014.Correlation between balloon release pressure
and no-reflow in patients with acute myocardial
infarction undergoing direct percutaneous coronary intervention. Chin Med J; 127 (6).
[6] Colombo A, Proietti R, Culic V, Lipovetzky N, Viecca M, Danna P. 2014.Triggers of acute myocardial infarction: a neglected piece of
the puzzle. J Cardiovasc Med (Hagerstown);
15(1):1-7.
[7] Madani M, Alizadeh K, Ghazaee S P, Zavarehee
A, Abdi S, Shakerian F, Salehi N, Firouzi A. 2013.
Elective Percutaneous Coronary InterventionThe
Relationship between Preprocedural BloodGlucose
Levels and Periprocedural Myocardial Injury. Tex
Heart Inst J ; 40(4):410-7.
[8] RODRIGO R, PRIETO J C and CASTILLO R
.2013.Cardioprotection against
ischaemia/reperfusion by vitamins C and E plus
n−3 fatty acids:molecular mechanisms and potential clinical applications. Clinical Science ; (124), 1–5.
[9] Di Filippo C, Cuzzocrea S, Rossi F, Marfella R, and
D’Amico M. 2006. Oxidative Stress as the Leading
Cause of Acute Myocardial Infarction in Diabetics. Cardiovascular Drug Reviews; 24 (2): 77–87.
[10] Trivelli L A . 1971. Quantitative determination of whole blood glycohemoglobin A1c. New Engl. J. Med., 284:353.
[11] Paglia D E and Valentine W N. 1967.Studies on the
quantitative and qualitative characterization of
potential key players in the development of cardiovascular disease (Da Silvaet al., 2013).
The fact that free oxygen radicals play a significant role during the cardiac IR is well known, being accompanied by superoxide dismutase and glutathione peroxidase depletion and reduction of the total antioxidant capacity that act as natural oxygen radical scavengers in the organism (Ghyasi et al.,2012). The antioxidant protection under the conditions of oxidative injury is a complex system in which the separate antioxidant elements co-operate with one another. The function of one antioxidant often potentiates the effects of another element in the system Ghyasi et al., (2012) also reported depletion in the activity of SOD and GPx in case of AMI.
In this work we measured the plasma glutathione
peroxidase (Pl GSHPx) as an important antioxidant; the
results indicated a significant reduction in its activity in
all studied groups compared to the healthy volunteers.
Nitric oxide is the main endothelial-derived vaso- dilator crucial for organ perfusion and coronary patency. Acute nitric oxide deficiency may lead to endothelial dysfunction with poor organ perfusion. Nitric oxide availability depends on the balance between a substrate (arginine) and an inhibitor of nitric oxide synthetase (asymmetric dimethylarginine). In patients with circulatory shock, a lack in nitric oxide as manifested by a low arginine:asymmetric dimethylarginine ratio correlates with other markers of circulatory dysfunction (cardiac index, lactate, pH, APACHE II) and is associated with higher in-hospital mortality, whether the origin of circulatory shock was cardiogenic or septic (Visser et al.,
2012).
Even in normal subjects, acute hyperglycemia causes
various changes like prolongation of corrected QT
interval and reduced nitric oxide availability (Giugliano et al., 1997 & Marfella et al., 2000).
This is in accordance with our results where the level of nitric oxide is significantly reduced in hyperglycemic group compared to normal control and also compared
with the AMI group.
Our results indicated a significant elevation in the
level of Hcy in all the studied patient groups compared to
the normal healthy one. Elevated Hcy levels on the day of
the MI have been reported by other authors (Remme et
al., 2001). Also our results are in accordance with Osorio et al., (2007) who reported a significant elevation in the level of Hcy in AMI in the first day of the onset of the infarction.
This study evaluated the diagnostic performance of certain oxidative markers, 8- isoprostane, AOPPs and CRP after the onset of the myocardial infarction in acute hyperglycemic patients. Our results indicated that both 8- isoprostane and AOPPs are early, highly sensitive and specific markers for the diagnosis of AMI. The study also confirmed the diagnostic value of sCRP and t-Hcy. NEFA yielded a significantly worse accuracy for diagnosing AMI.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 5, May-2014 121
ISSN 2229-5518
Biomarkers in Acute Cardiac Care and Implications for Strategic Management. Biomarker Insights; (8): 115–126.
[28] Singh V, Martinezclark P, Pascual M, Shaw ES, O’Neill WW. 2010. Cardiac biomarkers—the old and the new: a review. Coron Artery Dis. ;21(4):
244–56.
[29] McLean AS, Huang S J. 2012. Cardiac biomarkers
in the intensive care unit. Ann Intensive Care. ; (2):8.
[30] Loria V, Leo M, Biasillo G, Dato I, Biasucci LM.
2008. Biomarkers in Acute Coronary Syndrome.
Biomark Insights. ; (3): 453–68.
[31] Aldous SJ .2013. Cardiac biomarkers in acute
myocardial infarction. Int J Cardiol.; 164(3):282–94. [32] Gopcevic K, Rovcanin B, Kekic D, Radenkovic S.
2011. Matrix metalloproteinases and membrane damage markers in sera of patients with acute myocardial infarction. Mol Cell Biochem ;
(350):163–168.
[33] Kasap S, Gonenc A, Sener D E, Hisar I .2007. Serun
cardiac markers in patients with acute myocardial
infarction:oxidative stress, C-reactive protein and
N-terminal probrain natriuretic peptide.
J.Clin.Biochem.Nutr. ;(41):50-57. [34] LionelKR, JohnJ, SenN.2014.
Glycated hemoglobin A: A predictor of outcome in trauma admissions to intensive care unit. Indian J Crit Care Med; 18(1): 21-5.
[35] Cakmak M , Cakmak N , Cetemen S , Tanriverdi
H, EncY , Teskin O , Kilic I D . 2008. The value of admission glycosylated hemoglobin level in patients with acute myocardial infarction. Can J Cardiol ; 24(5):375-378.
[36] Mohanty P, Hamouda W, Garg R, Aljada A,
Ghanim H, Dandona P.2000. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab ; (85): 2970–2973.
[37] Dandona P, Chaudhuri A, Ghanim H, Mohanty
P.2007. Effect of hyperglycemia and insulin in acute coronary syndromes. Am J Cardiol ; (99):
12H–18H.
[38] Dandona P, Aljada A, Chaudhuri A, Mohanty P,
Garg R. 2005. Metabolic syndrome: a
comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation; (111): 1448–1454.
[39] Iwakura K, Ito H, Ikushima M, Kawano S, Okamura A, Asano K. 2003. Association between hyperglycemia and the no-reflow phenomenon in
patients with acute myocardial infarction. J Am
Coll Cardiol; (41): 1–7.
[40] FUJII H, SHIMIZU M, INO H, YAMAGUCHI M,
TERAI H, MABUCHI H, MICHISHIT, I, and
GENDA A .2002. Oxidative Stress Correlates with
Left Ventricular Volume after Acute Myocardial
Infarction. Jpn Heart J; (43): 203-209.
[41] Lv Z, Wub K, Chen X, Zhang X, Hong B. 2013.
Plasma intermedin levels in patients with acute
myocardial infarction. Peptides; (43): 121–125.
IJSER © 2014
erythrocyte GSHPx. J. Lab. Clin. Med; (70): 158-
169.
[12] Witko-Sarsat V, Friedlander M, Capeillère-Blandin
C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B.1996. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. May;
49(5):1304-13.
[13] Everse J, Kaplan NO.1973. Lactate dehydrogenases: structure and function. Adv Enzymol Relat Areas Mol Biol. ; (37):61-133.
[14] Takahashi M, Lee L, Shi Q, Gawad Y, Jackowski G.1996.Use of enzyme immunoassay for measurement of skeletal troponin-I utilizing
isoform-specific monoclonal antibodies. Clin
Biochem. ; 29(4):301-8.
[15] Gruber W. 1978. Inhibition of creatine
kinase activity by Ca2+ and reversing effect of
ethylenediaminetetraacetate. Clin Chem. ;
24(1):177-8.
[16] Meiattini F, Giannini G, Tarli P.1978. Adenylate
kinase inhibition by adenosine 5'-monophosphate
and fluoride in the determination of creatine
kinase activity. Clin Chem. ; 24(3):498-501.
[17] Trinder P. 1969. Determination of total serum cholesterol. J. Clin. Path., 22: 158.
[18] Richmond W. 1973. Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin Chem. ;
19(12):1350-6.
[19] Young D and Pestaner L. 1975. Determination of
serum triacylglycerols. Clin.Chem.;21:5.
[20] Friedewald W.T. 1972. Determination of high
desnity lipoprotein cholesterol. Clin. Chem.,
18:499.
[21] Lopes-Virella MF, Stone P, Ellis S, Colwell JA.1977.
Cholesterol determination in high-density
lipoproteins separated by three different methods.
Clin Chem. ; 23(5): 882-4.
[22] Henry R J .1974. Harper and Row (eds). In Clinical chemistry, principles and techniques, 2nd ed.
[23] Roberts WL, Sedrick R, Moulton L, Spencer A, Rifai N .2000. Evaluation of four automated high-sensitivity C-reactive protein methods:
implications for clinical and epidemiological applications. Clin Chem. ; 46(4):461-8.
[24] Miranda KM, Espey MG, Yamada K, Krishna M, Ludwick N, Kim S, Jourd'heuil D, Grisham MB, Feelisch M, Fukuto JM,Wink DA. 2001. Unique oxidative mechanisms for the reactive
nitrogen oxide species, nitroxyl anion. J Biol Chem.
; 276 (3):1720-7.
[25] Morrow JD.2000. The isoprostanes: their
quantification as an index of oxidant stress status
in vivo. Drug Metab Rev. ; 32(3-4):377-85.
[26] Refsum H, Ueland P M, Nygard O and Vollset S E.
1998. Homocysteine and cardiovascular disease.
Annu. Rev. Med.; (49): 31-62.
[27] Kossaify A, Garcia A, Succar S, Ibrahim A,
Moussallem N, Kossaify M and Grollier G,
Consortium S. 2013. Perspectives on the Value of
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 5, May-2014 122
ISSN 2229-5518
myocardial ischemic-reperfusion injury in male rat. J Res Med Sci.; 17(12): 1150–1155.
[49] Visser M, Vermeulen MA, Richir MC. 2012.
Imbalance of arginine and asymmetric dimethylarginine is associated with markers of circulatory failure, organ failure and mortality in shock patients. Br J Nutr ; 107(10): 1458–65.
[50] Giugliano D, Marfella R, Coppola R, et al.
1997.Vascular effects of acute hyperglycemia in humans are reversed by L-arginine. Evidence for reduced availability of nitric oxide during hyperglycemia. Circulation; (95): 1783–1790.
[51] Marfella R, Nappo F, De Angelis L. 2000. The effect of acute hyperglycemia on QTc duration in healthy
man. Diabetologia ; (43): 571–575.
[52] Remme WJ, Swedbergh K. 2001. Guidelines for the
diagnostic and treatment of chronic heart failure.
Eur Heart J; (22):1527–60.
[53] Osorio A, Ortega E, Ruiz-Requena E. 2008. Two models of homocysteine behavior in acute myocardial infarction. Clinical Biochemistry; (41):
277–281.
[42] Ekmekci A, Uluganyan M, Tufan F, Uyarel H, Karaca1 G, Kul S, Gungor B, Ertas G, Erer B, Sayar N, Gul M, Eren M .2013. Impact of admission blood glucose levels on prognosis of elderly patients with ST elevation myocardial infarction treated by primary percutaneous coronary intervention. Journal of Geriatric Cardiology; (10): 310-316.
[43] Havmoeller R, Reinier K, Teodorescu C, Ahmadi N, Kwok D, Uy-Evanado A, Chen YD, Rotter JI, Gunson K, Jui J, Chugh SS .2014. Elevated plasma free fatty acids are associated with sudden death: A prospective community-based evaluation at the time of cardiac arrest. Heart Rhythm.;
11(4):691-6.
[44] Cai H, Harrison DG. 2000. Endothelial dysfunction
in cardiovascular diseases: the role of oxidant
stress. Circ Res; 87(10):840–844.
[45] Crisby M, Kublickiene K, Henareh L, Agewall S
.2009. Circulating levels of autoantibodies to oxidized low-density lipoprotein and C-reactive protein levels correlate with endothelial function in resistance arteries in men with coronary heart disease. Heart Vessels ;(24):90–95.
[46] Polidori MC, Pratico D, Savino K, Rokach J, Stahl W, Mecocci P. 2004.Increased F2 isoprostane plasma levels in patients with congestive heart failure are correlated with antioxidant status and disease severity. J Card Fail. ; (10):334–338.
[47] Da Silva AS, Munhoz TD, Faria JL, Vargas-
Hérnandez G, Machado RZ, Almeida TC, Moresco RN, Stefani LM, Tinucci-Costa M. 2013. Increase nitric oxide and oxidative stress in dogs experimentally infected by Ehrlichia canis: effect on the pathogenesis of the disease. Vet Microbiol.; (164): 3-4.
[48] Ghyasi R, Sepehri G, Mohammadi M, Badalzadeh
R and Ghyasi A . 2012. Effect of mebudipine on
oxidative stress and lipid peroxidation in
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 5, May-2014 123
ISSN 2229-5518
Table (1): Statistics descriptive of different variables between patient groups and control group.
Test | Group | N | Mean | ± SD | Test | Mean | ± SD |
FBS (mg/dl) | Control | 20 | 89.35 | 6.29 | TnI ng/ml | 0.12 | 0.07 |
FBS (mg/dl) | AMI | 18 | ** 158.41 | 13 | TnI ng/ml | ** 11.04 | 2.27 |
FBS (mg/dl) | AMI with AHg | 20 | ** 294.47 | 69.32 | TnI ng/ml | ** 13.93 | 3.58 |
Hb A1c (%) | Control | 20 | 5.4 | 0.4 | NEFA (mmol/L) | 0.42 | 0.17 |
Hb A1c (%) | AMI | 18 | 6.34 | 0.61 | NEFA (mmol/L) | 0.52 | 0.23 |
Hb A1c (%) | AMI with AHg | 20 | ** 11.28 | 2.38 | NEFA (mmol/L) | 0.5 | 0.2 |
LDH IU/L | Control | 20 | 272.2 | 27.62 | Pl. GSHPx (mU/ml) | 203.51 | 20.71 |
LDH IU/L | AMI | 18 | 506.67** | 59.46 | Pl. GSHPx (mU/ml) | 73.93** | 14.31 |
LDH IU/L | AMI with AHg | 20 | ** 585.6 | 99.24 | Pl. GSHPx (mU/ml) | 67.37** | 9.64 |
CK-total IU/L | Control | 20 | 93.2 | 20.75 | NO | 46.21 | 3.31 |
CK-total IU/L | AMI | 18 | 233.5** | 43.63 | (µ mole/l) | 28.47** | 2.84 |
CK-total IU/L | AMI with AHg | 20 | ** 534.95 | 102.32 | 19.75** | 2.09 | |
CK-MB U/L | Control | 20 | 16.75 | 3.06 | sCRP mg/L | 6.42 | 0.29 |
CK-MB U/L | AMI | 18 | 49.18** | 14.53 | sCRP mg/L | 8.11* | 0.9 |
CK-MB U/L | AMI with AHg | 20 | ** 67.85 | 14.28 | sCRP mg/L | 17.19** | 2.92 |
Results are represented as mean ± S.D.
*Significant at p< 0.05; ** Highly Significant at p< 0.001 compared to control group.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 5, May-2014 124

ISSN 2229-5518
a b
c
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 5, May-2014 125
![]()
![]()
![]()
ISSN 2229-5518
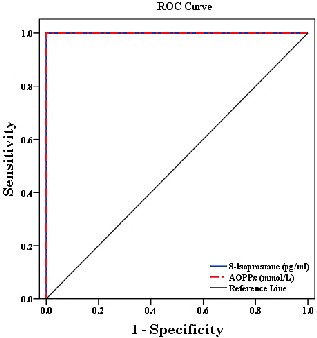
(ROC) curves displaying the accuracy of
8-Isoprostane & AOPPs for diagnosing
patients groups.
![]()
![]()
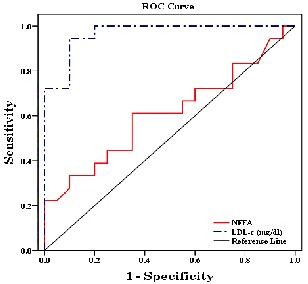
Groups.
Test Result Variable(s) | Area Under the Curve | Asymptotic Sig. | Cut off value |
NEFA (mmol/L) | 0.610 | 0.248 | 0.465 |
LDL-c (mg/dl) | 0.967 | <0.001 | 127.91 |
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 5, May-2014 126
ISSN 2229-5518
![]()
![]()
![]()
Groups.
Test Result Variable(s) | Area Under the Curve | Asymptoti c Sig. | Cut off value |
sCRP (mg/L) | 0.958 | <0.001 | 7.15 |
t-Hcy (mmol/L) | 0.925 | <0.001 | 10.54 |
Authors details:
Biochemistry Department, Faculty of Science, Ain Shams University (Drs. Hassan, Tawfik And Borai) Al-Hussein hospital ,Al-Azhar university, Cairo, Egypt (Dr.Mahran)
Corresponding author (*)
E- Mail Address: nana_samir@hotmail.com
IJSER © 2014 http://www.ijser.org