molar mass of urea(M = 60.06 g/mol), n is the
number of electrons exchanged during reaction (1),
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1715
ISSN 2229-5518
1Mathematical and Physics Department, Faculty of Engineering, Mansoura University, Egypt
2Chemical Engineering Department, Faculty of Engineering, Cairo University, Egypt
Abstract: In this study, experiments were carried out using a bench-scale electrochemical cell incorporating flow-by porous graphite electrodes for oxidation of human urine was collected from a mosque during baths on urea were analyzed and found that the concentration ranges of of urea between 50 to 100 ppm . The effect of anodic current density, and influent feed flow rate, on basic process indices, the removal rate of urea, and current efficiency, and energy consumptions, were investigated.
The experimental results showed that, the removal rates of urea increased with increasing the current density; at the same time, the energy power consumption increased, and the current efficiency decreased. At human urine concentration of 100 ppm, the maximum current efficiency is 27, the maximum removal rate is 0.33 g/h, and minimum energy consumption is 196 kWh/kg.
Key words: Urea, flow-by, graphite, porous, decomposition
1.Introduction
One of the effective measures to help solve the water shortage problem involves recycling of human urine for its use as flush water this is because while an adult passes approximately 1.5 L of urine per day, 10 times
by urease. As a result of the electrochemical reaction, the reactivity of urease was found to be suppressed when the potential was maintained at 240 mV vs SHE (Standard Hydrogen Electrode). Thus, urine can be stored under continuous electrochemical
IJSER
this amount water is used simply to flush this.
Therefore, a large amount of water can be saved (approximately 20 L per person daily) if all the urine is recycled [1].
Electrochemical waste destruction shows several benefits in terms of costs and
safety. The process runs at very high electrochemical efficiency and operates essentially under the same conditions for a wide variety of wastes. Operation at room temperature and atmospheric pressure reduces the possibility of volatilization and the discharge of unreacted waste. The waste treatment can be terminated within seconds by simply cutting off power to the electrodes.
One of the common water pollutants is urea which is usually present in wastewater
discharges from several sources. Urea is not
directely toxic but its hydrolysis into ammonia leads to toxicity to both animal and marine life. Urea management has been a major environmental and health issue. Human/animal urine, industrial synthesis process of urea, and dialysate used in artificial kidney, produce a large amount of wastewater with varying urea concentration. The wastewater containing urea can go through a natural conversion to ammonia, which is then
emitted to the atmosphere [2].
Urea was electrochemically treated in order to suppress the emission of the unpleasant odor due to ammonia, which is an end product of the hydrolysis of urea in urine
treatment without emitting the unpleasant
ammonia odor and thus serve as toilet flush water [1].
The electrochemical treatment for urea-rich wastewater has recently become a topic of attention
due to its potential applications, including wastewater remediation, hydrogen production, electrochemical sensors, and fuel cells [3-6].
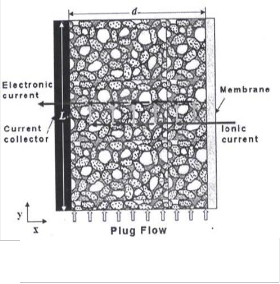
Urea-rich wastewater has also been identified as a good source for hydrogen production in alkaline medium. The major constituent of human or animal waste on earth is urine, containing about 2–2.5 wt.% of urea suggesting the availability of a considerable amount of urea in municipal wastewater. Also, a large amount of wastewater with varying concentrations of urea is produced during the industrial synthesis of urea[7]. Urea electrolysis would be an efficient way for hydrogen production from urea-rich wastewater thus potentially using urine [7-11], the product of human/animal excretion as an energy source, as shown in Fig. 1. Urea is electrochemically oxidized at the anode producing N2 and CO2 , whereas pure hydrogen is evolved at the cathode that can be collected as a valuable fuel and clean water is obtained as a by-product [7].
The anodic oxidation of urea with simultaneous evolution of hydrogen at the cathode can be represented by the following overall cell reaction: CO (NH2) 2(aq) + H2O (l) → N2 (g) + 3H2 (g) + CO2 (aq)
(1)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1716
ISSN 2229-5518
The electrode reactions, whether in acidic or alkaline medium, would involve 6 electrons.
It is clear from the previous literature review that no attempt has been done to study the electrochemical removal of urea from wastewater on bench-scale level. Unlike phenol[12,13],aniline[14,15], and several other
organic pollutants, urea electrochemical removal has not be subjected to extensive study beyond lab-scale level. The relatively low concentrations of urea in industrial wastewater necessitate the use of flow- through or flow-by electrodes to avoide mass- transfer limitations. These electrodes have been used in several applications[16-19] since they provide high mass-transfer coefficients and large electrode surface areas.
The three-dimensional porous electrodes offer particularly high values of the electro active area per unit reactor volume and give a moderate increase in mass transport coefficient. The result is a significantly increased performance from a given volume of reactor, compared to two-dimensional electrode materials.
Flow-by porous electrode work as flow-
was added to obtain the desired pH and it was stored at 4 °C until use.
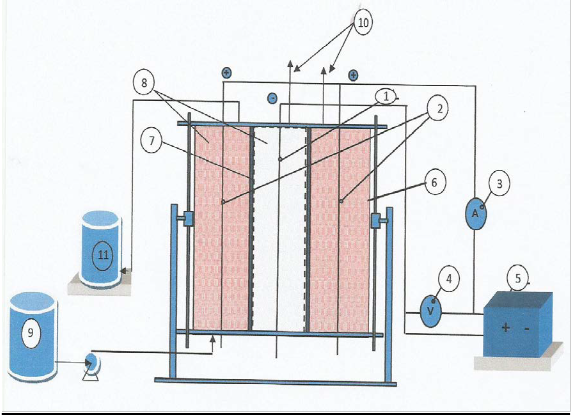
A schematic of the experimental setup is shown
in Fig. 2.
The porous electrodes were contained in a vertical Plexiglas cylinder of internal diameter of
20 cm, and height of 40 cm, with two end flanges made from PVC. The anode compartment was filed by graphite to a height of 27 cm.
A stainless steel screen (mesh 5) in the form of a cylinder with internal diameter of 10 cm was used as the cathode compartment. The anode compartment was the annular space between that stainless steel cylinder and the cell body.
Three stainless steel rods of 10 mm diameter and 50 mm length were used; two of them as current collectors in the anode compartment, while
the third one in the cathode compartment.
Graphite powder with the screen analysis shown in Table 1 was poured into the anodic compartment, while graphite granules with 0.5 cm average particle diameter into the cathode compartment. The stainless steel cylinder was enclosed by a polyamide membrane to minimize any transfer of graphite between both
IJSER
through porous electrode, but the difference
between them is the electricity flow, which is perpendicular to that of electrolyte in case of flow-by and parallel in case of flow-through as shows in Fig.1 [20].
Trainham and Newman[20] published a comparison between flow-through and flow- by electrodes. They concluded that economically the flow-by electrode is superior than flow-through.
The advantage of the porous electrode lies in the high rates of reaction, and consequently reaction is usually arranged to be under limiting-current conditions which corresponds to the maximum rate of mass
transfer [20].
In this study, experiments were carried out using a bench-scale electrochemical cell incorporating flow-by porous graphite electrodes for decomposition of urea. The effects of current density, feed flow rate, composition of the electrolyte, and urea concentration in influent stream, on the removal rates, current efficiency and energy consumptions were investigated.
Human urine was collected from a mosque
during baths on urea were analyzed and found that the concentration ranges of of urea between 50 to
100 ppm .
The investigated urine solutions were prepared by dissolving sodium chloride into human urine to obtain the desired concentration, while sulfuric acid
compartments and to maintain electric separation
between both compartments.
The feed entered through an opening at the bottom of the anode compartment, while the outlet flow and the gas vents were located at the upper flange of the anode and cathode compartments respectively.
A dosing pump of type Master Flex Cole- Palmer Instrument Company controlled the feed flow rate between 0.56 and 1.9 ml/s.
The cell was connected to laboratory DC power supply Model (GPR-1810 HD) that can supply current up to 10 A at potential up to 18 V. The potential and current were measured using digital multi-meters of type METEX M-3800.
The characteristics of raw and treated water
were determined by measuring the following parameters:
1- pH measured by using pH meter, model AD
1030 pH/mV
2- Urea concentration measured by using
Spectrophotometer Genesys 105 UV-VIS [21,
22].
Human urine was fed to the anode compartment with controlled flow rate using the
dosing pump. Triplicate effluent samples were collected in special bottles after 100, 120 and 140 minutes to ensure that steady state conditions were reached. The current and potential of the cell were recorded at the same sampling time. The average of the concentrations of the three samples was considered as the effluent concentration for each
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1717
ISSN 2229-5518
run. The temperature was adjusted using an electric heater with variable heat input and thermometer placed in the feed solution storage vessel.
The investigated parameters were: the
apparent anodic cell current density (J = 1.8 – 7.8 mA/cm2),and influent flow rate(Q = 0.56 – 1.9 ml/s The results of the above measurements
were used to calculate the removal rates, removal efficiency, current efficiency, and energy consumption for electrochemical oxidation of human urine:
The removal rate of urea was calculated from the following equation;
R = Q (ci - co ) (10-6) (3600) (2)
Where R is the removal rate of human urine in
g/h on the surface of graphite anode, Q is the flow rate of influent in ml/s, and ci and co are the concentrations of urea in influent and effluent in ppm, respectively.
The removal rate of human urea per unit volume of anodic compartment was calculated from the following equation;
Where Aanode is area of anodic compartment in
At flow rates up to 1.6 ml/s, the removal rate of urea continuously increased due to the increase in mass-transfer coefficient. The change in the removal rate was negligible when the flow rate was increased from 1.6 to 1.9 ml/s. This is due to two opposing effects; the increase in mass-transfer coefficient due to the higher flow rates, and the decrease of residence time available for urea removal.
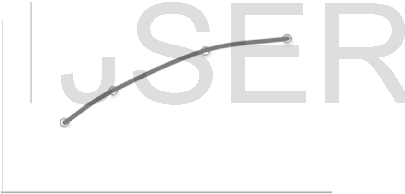
As shown in Fig. 4 to 6, increasing the
current density, increased the rate of urine removal due to increase of the rate of urea oxidized on the anode, providing that the mass-transfer rate is high enough. However, above 5.6 mA/cm2 the change in the removal rate was negligible as limiting current density is approached, where the interfacial concentration of urea on the graphite anode dimishes practically to zero.
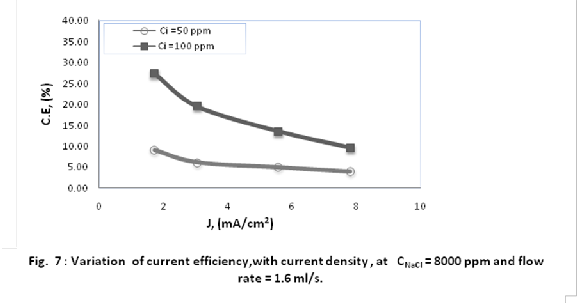
As indicated in Fig. 7, an increase in anode current density from 1.78 to 7.8 mA/cm2 at flow rate of 1.6 ml/s, and best sodium chloride concentration 8000 ppm[23] caused decrease in current efficiency from 27 to 9 % and from 9 to 4 % for human urine
cubic meter.
IJSEconcentrationRof 100 and 50 ppm,
The theoretical current was calculated from the
following equation;
Ith = Q (ci - co )(n)* (96500)(M/1000,000) ( 4 )
Where Ith is the theoretical current in A, M is the
molar mass of urea(M = 60.06 g/mol), n is the
number of electrons exchanged during reaction (1),
n = 6 and F is the Faraday constant (Ah/mol).
The current efficiency (C.E) of urea was calculated from the following equation;
C.E = (Ith. / Iact )* 100 (5)
Where, Iact the actual current measured in A.
The energy consumption was calculated from the
following equation;
Z = (V * Iact ) / R (6)
Where, Z is the energy consumption in kWh/kg of
urea removed and V is the cell potential in volt.
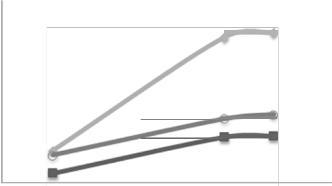
Fig. 3 shows the variation of removal rate of
urea with influent flow rate for different concentrations of human urine. It is obvious that the removal rate of urea increases with the increase in influent flow rate. This trend agrees with results of removal of urea from industrial wastewater
using electrochemical oxidation[23] who used
graphite anode for electrochemical oxidation of urea. Also this trend agrees with the results of De Sucre and Watkinson[24] who also used lead dioxide for anodic oxidation of phenol for wastewater treatment and operating both in batch and continuous modes.
respectively.
For the same conditions, the energy consumption increased from 35 to 196 kWh/kg for human urine concentration of 100ppm.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1718
ISSN 2229-5518
Sieve no | Screen opening µ m | Average particle diameter, Cm | Mass fraction retained |
20 30 45 60 100 200 | 850 600 355 250 150 75 | - 725 × 10-4 477.5 × 10-4 302.5 × 10-4 200 × 10-4 112.5 × 10-4 | 0.0623294 0.1613734 0.419561 0.2739 0.075073 0.0052507 |
IJSER
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013
ISSN 2229-5518
1719
0.40
0.35 Ci = 100ppm
0.30 Ci = SO ppm
0.25 Ci=50ppm
L
......... 0.20
III.J
a:. 0.15
0.10
0.05
0.00
0.25 0.75 1.25 1.75 2.25
Flow rate, (ml/s)
Fie. 3 : Variation of remova l rate of urea decomposed(R) with flow rate, at
C,.,. 0 = 5000 ppm, bed hieht = 27 em, and current density J= 5.6 mA/cm 2
0.35
0.30
L 0.25
.........
IlL)
a:.' 0.20
0.15
0.10
0
--e-Q = 1.6 ml/s
2 4 6 8
J, (mA/Cm 2 )
Fie. 4 : Variation of removal rate of urea decomposed,( R) wit h current density, at
C; = 100 ppm, and Cuacl = 8000 p pm .
IJSER © 2013
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013
ISSN 2229-5518
1720
Q= L6ml/s
e
IlL)
a;'
0.10
0.08
0.06
0.04
0.02
0
2 4 6 8
J, (mA/Cm 2 )
Fie. S : Variation of removal rate of urea decom posed,( R} with current
density, at C1 = 80 ppm, and C u:ac 1 = 8000 ppm .
........
IlL)
0.14
0.12
0.10
0.08
Q =L6ml/s
a; 0.06
0.04
0.02
0.00
0

2 4 6 8
J, ( m A/Cm 2 )
Fie. 6 : V ariation of removal rate of urea decomposed,( R } with current density, at
ci =50 ppm, a nd CraC I = 8000 ppm .
IJSER © 2013
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1721
ISSN 2229-5518
IJSER
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1722
ISSN 2229-5518
-The advantage of the porous electrode lies in the high rates of reaction, and consequently reaction is usually arranged to be under limiting conditions.
- In this study, it was proved that using flow- by porous graphite electrode for the treatment of human urine, for its use as flush water, is an effective method.
- For it is recommended to use an influent flow rate of 1.6 ml/s at a current density 5.6 mA/cm2, electrolyte concentration 8000 ppm, height of bed 27 cm, and pH 4 . At these conditions the removal rates of urea was
0.33,and 0.1 g/h at current efficiencies of 14, and 6 % and energy consumption of129, and344 kWh/kg for initial concentrations of
100, and 50ppm, respectively.
-The three of proposed cell can be introduced in the treatment of wastewater containing urea coming out of camps located far from civilian life or marble quarry.
hydroxide catalysts for urea electro- oxidation", ElectrochimicaActa, Vol. 61,
2012, pp.25-30.
6. Dan Wang,Wei Yan,Gerardine G, 2012," Enhanced electrocatalytic oxidation of urea based on nickel hydroxide nanoribbons", Journal of power sources, Vol. 217, 2012, pp.
498-502.
7. Dan Wang, Wei Yan,Santosh H. Vijapur, Gerardine G,2013," Electochemically reduced graphene oxide-nickel nanocomposites for urea electrolysis", ElectrochimicaActa, Vol.
89, 2013, pp. 732-736.
8. Kirk, D. W., and Sharifian, H., “ Electrochemical Oxidation of Phenol “ Journal of Electrochemical Society, pp. 921-
924, vol. 133, No. 5, 1985.
9. Guido Busca, Silvia Berardinelli, Carlo Resini,2008." Technologies for the removal of phenol from fluid streams: A short review of recent developments", Journal of Hazardous Materials, Vol.160, 2008,pp. 265–288.
10. Kirk, D. W., Sharifian, H., and Floukes, F. R., “ Anodic Oxidation of Aniline for Wastewater Treatment “ Journal of applied
IJSER
-This cell can be used in the future in
industrial effluent Treatment with small quantities of fertilizer factories during working day.
- This cell can be used in the future in dialyses process effluent Treatment before ditched in drainage systems.
1. MineoIkematsu, Kazuhiro Kaneda, Masahiro
Iseki, Masashi Yasuda,
2007,"Electrochemical treatment of human urine for its storage and reuse as flush water", Science of the Total Environment, Vol. 382,
2007, pp. 159–164. [1]
2. WojciechSimka , Jerzy Piotrowski,
GinterNawrat, Influence of anode material on electrochemical decomposition of urea, ElectrochimicaActa 52 (2007) 5696–5703[2]
3. Vedasri Vedharathinam, Gerardine G. Botte,
2012, "Understanding the electro-catalytic oxidation mechanism of urea on nickel electrodes in alkaline medium",Electrochimica Acta, Vol
81,2012,PP 292-300.
4. LjubicaMatijasevic, Igor Dejanovic , Hrvoje Lisac,2010," Treatment of wastewater generated by urea production", Conservation and Recycling , Vol. 54 , 2010 149–154
5. Wei Yan, Dan Wang, Gerardine G.
Botte,2012," Nickel and cobalt bimetallic
Electrochemistry, pp. 285-292, vol. 15, 1985.
11. Yijiu Li, Feng Wang, Guoding Zhou, 2003, " Aniline degradation by electrocatalytic oxidation", Chemosphere, Vol.53, 2003, pp.1229-1234.
12. Kirk, D. W., and Sharifian, H., “ Electrochemical Oxidation of Phenol “ Journal of Electrochemical Society, pp. 921-
924, vol. 133, No. 5, 1985.
13. Guido Busca, Silvia Berardinelli, Carlo
Resini,2008." Technologies for the removal of phenol from fluid streams: A short review of recent developments", Journal of Hazardous Materials, Vol.160, 2008,pp. 265–288.
14. Kirk, D. W., Sharifian, H., and Floukes, F. R., “ Anodic Oxidation of Aniline for Wastewater Treatment “ Journal of applied Electrochemistry, pp. 285-292, vol. 15, 1985.
15. Yijiu Li, Feng Wang, Guoding Zhou, 2003, " Aniline degradation by electrocatalytic oxidation", Chemosphere, Vol.53, 2003, pp.1229-1234.
16. Development of a 1 kW h Zn-C12 battery system, Rept. EM-249, Electric Power ResearchInstitute, 1976
17. S. Mitra, J. Power Sources, 1982, 8, 359
18. J. Jorne, J. Electrochem. Soc., 1982, 129,
2251.
19. R. Yu. Bek and A. P. Zamyatin, Elektrokhimiya, 1978, 14, 1196.
20. J. A. Trainham and J. Newman, Electrochim.
Acta, 1981, 26, 455.
21. R. Yu. Bek and A. P. Zamyatin,
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1723
ISSN 2229-5518
Elektrokhimiya, 1978, 14, 1196.
22. YoungDS.Effects of disease on clinical lab.Tests.4th ed AACC2001.
23. Mahmoud H. Mahmoud, Nabil M. Abdel- Monem , Omar E. Abdel-Salam, Ahmed F. Nassar,2013” Removal of urea from industrial wastewater using electrochemical decomposition”, Journal of Life Science
2013;10(3)
24. De Scure, V. S., and Watkinson, A. P., “ Anodic Oxidation of Phenol for Wastewater treatment “ the Canadian Journal of Chemical Engineering, pp. 52-59,vol. 59, 1981. Burtis A etal.Tietz Textbook of Clinical Chemistry,
3rd ed AACC 1999.
IJSER
IJSER © 2013 http://www.ijser.org