
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1266
ISSN 2229-5518
Optimization of the Operating Conditions of Turbidity Removal from Synthesized Dairy Wastewater Using Pumpkin Seed as a
Coagulant
Blessing Mike JOE1, Saidat Olanipekun GIWA2*, Maryam IBRAHIM3, Yusuf Olabode RAJI4, Abdulwahab GIWA5
1,2,3,4Chemical Engineering Department, Faculty of Engineering and Engineering Technology, Abubakar Tafawa Balewa University, Tafawa Balewa Way, P.M.B. 0248, Bauchi, Nigeria
5Chemical and Petroleum Engineering Department, College of Engineering, Afe Babalola University, KM. 8.5, Afe Babalola Way, Ado-Ekiti, Ekiti State, Nigeria
Emails: 1blessingmike.21961@ gmail.com; 2*sogiwa@ atbu.edu.ng; 3maryamibrahim2704@gmail.com; 4yoraji@atbu.edu.ng; 5agiwa@abuad.edu.n g
*Corresponding author
—————————— ——————————
Wastewater from industries varies so greatly in both flow and pollution strength. It is impossible to assign fixed values to their constituents. Industrial wastewaters may contain suspended, colloidal and dissolved (mineral and organic) solids. In addition, they may be either excessively acidic or alkaline and may contain high or low concentrations of coloured matter. These wastewaters may contain inert, organic or toxic materials and possibly pathogenic bacteria (Alturkman,
2006). As such, they must be treated or processed to
make their concentrations be within acceptable limit
before being released into the environment or water
bodies. Treatment of these wastewater types is an
important issue in environmental protection as the wastewater normally contains pollutants that, if not efficiently treated, can cause serious hazards to the environment.
The food processing industry seeks cost- effective reduction and recycling technologies for food processing wastewaters due to the enforcement of wastewater discharge regulations and escalating sewage surcharges. These technologies include both source reduction options (technologies to reduce the amount of water used) and treatment options (technologies to reduce the amount or contamination level of wastewaters requiring discharge) (Philips, 1997).
Over the last few decades, several methods have
been applied to treatment of industrial wastewater;
these include membrane separation, solvent extraction, chemical reduction, chemical precipitation, evaporation, lime coagulation, cementation, ion exchange, reverse osmosis and electrodeposition (Singh and Kaushal,
2013). However, of all these methods, the commonly used ones are pre-liming, coagulation, flocculation, sedimentation and filtration.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1267
ISSN 2229-5518
The production of potable water from most raw
water sources usually entails the use of a
coagulation/flocculation stage to remove turbidity in the
form of suspended and colloidal materials. This process plays a major role in industrial wastewater treatment by reducing turbidity, bacteria, algae, colour, organic compounds, and clay particles. The presence of suspended particles could clog filters or impair disinfection process, thereby dramatically maximizing the risk of waterborne diseases. Many coagulants, one of which is alum, are widely used in conventional water treatment processes, based on their chemical characteristics. These coagulants are classified into inorganic, synthetic organic polymers, and natural coagulants (Vara, 2012).
Alum has been the most widely used coagulant because of its proven performance, cost effectiveness,
relatively easy handling and availability. Recently, much attention has been drawn to the extensive use of alum. Aluminium, which is contained in alum, is regarded as an important poisoning factor in dialysis encephalopathy. It (aluminium) is one of the factors which might contribute to Alzheimer's disease. Some synthetic organic polymers such as acrylamide have neurotoxicity and strong carcinogenic effect. Besides, the use of alum salts is inappropriate in some developing countries because of the high costs of imported chemicals and low availability of chemical coagulants (Vara, 2012). It, therefore, means that there is the need to embark on the use of natural coagulants for water
treatment because the use of chemical coagulants such
as aluminium salt to treat industrial wastewater has
been found to be harmful to human health.
Furthermore, a number of studies have pointed out that the introduction of natural coagulants as substitutes for metal salts may ease the problems associated with chemical coagulants. Natural macromolecular coagulants are promising and have attracted the attention of many researchers because of their abundant source, low price, multi-purposeless, and biodegradation. Okra, rice, and chitosan are natural compounds which have been used in turbidity removal. The extracts of the seeds have been mentioned for drastically reducing the amount of sludge and bacteria in sewage (Vara, 2012). Other used natural coagulants include Nirmali seed and maize, mesquite bean and Cactus latifari, Moringa oleifera seed, and pumpkin seed. The main advantages of using plant-based coagulants as water treatment materials are apparent; they are cost- effective, unlikely to produce treated water with extreme pH and highly biodegradable. Naturally occurring coagulants, such as pumpkin seed, are usually presumed safe for human health (Birima et al, 2013).
Pumpkin (the seed of which is shown in Figure 1)
is one of the most delicious vegetables. It is commonly
used in cooking various dishes. This pulpy vegetable
has many seeds that are often thrown away as most of us are unaware of their wonderful taste and health benefits (Reddy, 2014).
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1268

ISSN 2229-5518
Figure 1: Pumpkin seed (Pepitas, Cucurbita pepo) (http://www.whfoods.com/genpage.php?tname=foodspice&dbid=82)
Pumpkin seeds also have water soluble proteins
that are released from the powdered seed that attach themselves and bind between the suspended particles and, thereby, forming larger agglomerated solids. These flocculated solids would be allowed to settle prior to boiling and subsequent consumption of the water. The active fraction of the seeds has received much interest. Investigations by professor Tauscher at the University of Karlsruhe, Germany have revealed that the coagulant properties of the seeds are due to a series of low molecular weight cationic proteins. The potential toxicity of the seeds has been considered in two major studies; the conclusion of both were that the doses typically used for water treatment posed no serious threat to human health (Ali et al., 2010). As such, pumpkin seed can be used as a coagulant for the treatment of industrial wastewater because it is not harmful to the health, readily available, accessible and affordable.
Some researchers have worked on the use of
natural coagulants for water treatment. Among them is the work of Ndabigengesere (1995) that studied the active agents and mechanism of coagulation of turbid waters using Moringa oleifera. He was able to discover that the active agent in Moringa oleifera extracts were diametric cationic proteins, and that the mechanism of coagulant with Moringa oleifera appeared to consist of adsorption and neutralization of the colloidal charges compared to alum. Also, Diaz et al. (1999) carried out a
preliminary evaluation of turbidity removal by some
natural coagulants (Cactus latifaria and seeds of Prosopis
juliflora) and found out that both Cactus latifaria and
seeds of Prosopis juliflora were effective in reducing the
pollutant of the water they considered to the required standard of 5 NTU from both high (100-200 NTU) and low (30-40 NTU) turbidity ranges.
Generally, the factors affecting the coagulation
process of pumpkin seed include coagulant dose,
coagulation time and pH. These factors affect the
manner in which the seed is used to treat water.
Therefore, it is necessary to obtain their values that will treat water to its best. In other words, the optimum parameters of the factors need to be obtained and used to, properly, carry out water treatment. One of the methods obtained from the literature that can be used for optimizing a process like is the response surface methodology.
Response surface methodology (RSM) is a widely used technology for rational experimental design
and process optimization in the absence of mechanistic information (Box and Draper, 1987; Myers and Montgomery, 1995; Giwa and Giwa, 2012). RSM initiates from Design of Experiments (DoE) to determine the values of the factors to be used for conducting experiments and collecting data. The data are then used to develop an empirical model that relates the process response to the factors. Subsequently, the model facilitates to search for better process response, which is
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1269
ISSN 2229-5518
validated through experiment(s). The above procedure
iterates until an optimal process is identified (Chi et al.,
2012; Giwa and Giwa, 2012). RSM has seen diverse
applications in almost every area of scientific research and engineering practice, including the development of chemical and biochemical processes (Agatonovic- Kustrin et al., 1998; Baumes et al., 2004; Dutta et al.,
2004; Hadjmohammadi and Kamel, 2008; Shao et al.,
2007; Tang et al., 2010; Yan et al., 2011a,b; Giwa and Giwa, 2012). It is perceived that this good method of optimization can be extended to wastewater treatment.
Therefore, the aim of this work is to obtain the
optimum conditions for treating synthesized dairy
wastewater in order to remove its turbidity using a
coagulant developed from pumpkin seed by employing
the central composite design of the response surface
methodology of the Design Expert. The optimum values obtained using the response surface methodology will be compared to those obtained from the conventional jar experimental method.
Given in Figure 2 below is the flowchart of the steps involved in preparing the pumpkin seed coagulant used in this work.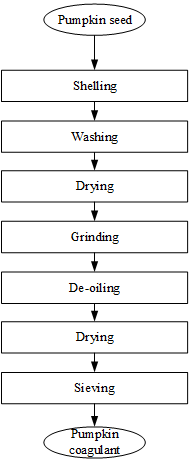
Figure 2: Flowchart for coagulant preparation
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1270
ISSN 2229-5518

Figure 3: Soxhlet Extractor
In preparing the coagulant from pumpkin seed,
moist seed was obtained from Muda Lawal Market in Bauchi, Bauchi State of Nigeria and shelled, washed and dried in an oven at a temperature of 50 oC for about 7 hrs. After that, the seed was grinded and placed in a 250 ml Soxhlet extractor (Figure 3) operated at 44 oC for 8 hr for its oil content to be extracted. During the oil
extraction operation, n-hexane was used as the solvent.
The resulting cake, shown in Figure 4, obtained after drying the pumpkin seed powder from which oil had been extracted for 5 min at 50 oC and sieving yielded the powder used as the coagulant for the treatment of the dairy wastewater that was synthesized in this work.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1271
ISSN 2229-5518
Figure 4: Pumpkin seed cake
The synthetic dairy wastewater used in this
research work was prepared by dissolving instant powdered milk in tap water. The solution was thoroughly shaken and left at room temperature in a tightly covered container for about 24 hr.
The turbidity of the synthesized dairy
wastewater was measured before and after the treatment (experiment) using DR/890 Colorimeter. A pH meter was also used to measure the pH of the synthesized wastewater. Prior to each experiment, pH adjustment was done by adding 0.5 M H2 SO4 or NaOH, as the case demanded, to the wastewater.
A jar test apparatus (Flocculator SW6) with six beakers was used to find the optimum coagulant dose that would give the minimum residual turbidity or the maximum turbidity removal. In order to achieve this,
500 ml of the synthesized dairy wastewater was poured into each of the beakers labelled from 1-6 and coagulant dose was varied between 1 and 6 g/l. The coagulation was carried out for 15 minutes out of which the first 5
minutes were used for rapid mixing at a speed of 150
rpm, while at the last 10 min of the coagulation, the mixture was moderately mixed at 80 rpm. After the coagulation process, the mixture was allowed to settle for 30 minutes and filtered to obtain the treated wastewater.
In this aspect of the work, with the aid of Design Expert 7.0.0 (Stat-Ease, 2005), a set of 20 experiments, as shown in Table 3, was generated according to central composite design of the response surface methodology using alpha value of 1.6818. The factors and their actual and coded values are as given in Table 1. The experimental results obtained after the experiments were analysed using a full quadratic model that was later modified to improve the significance and the prediction capability of the developed model. The response (residual turbidity) was then minimized using the numerical optimization approach of Design Expert to obtain the optimum operating conditions of the dairy wastewater treatment process.![]()
Table 1: The ranges of the experimental factors![]()
Factors | Symbols | -α | -1 | 0 | +1 | +α |
pH | A | 5.5 | 6.11 | 7 | 7.89 | 8.5 |
Coagulant dose, g/l | B | 1.0 | 1.81 | 3.0 | 4.19 | 5.0 |
Coagulation time, min | C | 5 | 6.01 | 7.5 | 8.99 | 10 |
![]()
The wastewater used in this work was synthesized, and after the synthesis, it was analysed to get its initial characteristics. It was obtained from the initial analyses of the synthesized wastewater that its pH was 6.84 while its turbidity was 467 NTU. Even though the pH of the wastewater was approximately equal to that of the water that could be discharged into the water body directly, its turbidity was found not to be friendly. This was what, actually, necessitated the need for the treatment. The first treatment (jar test) given to the wastewater was carried out by varying the coagulant dose in order to see its effects on the removal of the turbidity of the water.
Shown in Table 2 are the results obtained from
the jar experiments carried out in which the pumpkin seed coagulant dose was varied while the other factors (pH and coagulation time) were kept constant, in order
to determine the dose that could give the lowest turbidity of the water being considered. From the results, it was observed that increase in coagulant dose led to a corresponding decrease in turbidity, and the optimum dose of the coagulant was observed to be 4 g/l because that was the dose that was used to achieve a minimum turbidity of 135 NTU. An increase in the coagulant dose after that optimum dose of 4 g/l was found to result in an increase in the turbidity of the water. Under the optimum conditions (that is, pH of
6.84, coagulation time of 15 min, and coagulant dose of
4.0 g/l), the final turbidity percentage removal was
calculated to be 71.09%. This value of the percentage turbidity removal was found not be too bad, but it was desired to improve it a bit. As such, a better statistical method of optimization, known as response surface methodology, which varies all the parameters to obtain the optimum values was employed.
Table 2: Jar experimental results
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1272
![]()
ISSN 2229-5518
![]()
Run | pH | Coagulant dose (g/l) | Coagulation time (min) | Residual turbidity (NTU) |
1 | 6.84 | 1.0 | 15 | 290 |
2 | 6.84 | 2.0 | 15 | 286 |
3 | 6.84 | 3.0 | 15 | 215 |
4 | 6.84 | 4.0 | 15 | 135 |
5 | 6.84 | 5.0 | 15 | 224 |
6 | 6.84 | 6.0 | 15 | 316 |
![]()
The results of the experiments designed, with
the aid of response surface methodology, and using the
central composite design, and carried out for testing the
coagulation and water treatment capability of the pumpkin seed coagulant developed are given in Table 3. According to the table, the turbidity removal of the pumpkin seed coagulant was found to be affected by the variations in the input factors (pH, coagulation time and coagulant dose). For instance, the experiment carried out at minimum values of all the factors (pH of 6.11, coagulant dose of 1.81 g/l and coagulation time of 6.01 min) was found to yield water with turbidity of 273
NTU (Run 19) while the one performed at the maximum values of the factors (pH, coagulant dose and coagulation time of 7.89, 4.19 mg/1, 8.99 min,
respectively) gave water with residual turbidity of 194
NTU (Run 9). This implies that as the factors of the
experiments were increased from minimum to
maximum values, there was a decrease in the turbidity and, thus, an increase in the percentage turbidity removal. Also, at the centre point of the experiments (that is, pH = 7, coagulant dose = 3 g/l, coagulation time
= 7.5 min), the average residual turbidity was found to
have a value of 252 NTU (runs 20, 2, 16, 8, 7, 11). It has, thus, been found that the average residual turbidity value obtained from the centre point experiments was less than that obtained at the minimum points but higher than that produced by the maximum values of the factors used in the experiment.![]()
Table 3: Experimental factors and responses![]()
![]()
1 5.50 3.00 7.50 224 52.0343
2 7.00 3.00 7.50 250 46.4668
3 7.00 3.00 5.00 192 58.8865
4 7.00 5.00 7.50 132 71.7345
5 6.11 4.19 6.01 203 56.5310
6 6.11 4.19 8.99 163 65.0964
7 7.00 3.00 7.50 250 46.4668
8 7.00 3.00 7.50 256 45.1820
9 7.89 4.19 8.99 194 58.4582
10 8.50 3.00 7.50 232 50.3212
11 7.00 3.00 7.50 256 45.1820
12 7.00 3.00 10.00 197 57.8158
13 7.89 1.81 8.99 367 21.4133
14 7.89 1.81 6.01 266 43.0407
15 6.11 1.81 8.99 237 49.2505
16 7.00 3.00 7.50 250 46.4668
17 7.89 4.19 6.01 184 60.5996
18 7.00 1.00 7.50 320 31.4775
19 6.11 1.81 6.01 273 41.5418![]()
20 7.00 3.00 7.50 250 46.4668
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1273
ISSN 2229-5518
Furthermore, increasing the pH of the
wastewater (run 10) and coagulation time (run 12)
beyond their normal maximum values for the experiment resulted in increase in residual turbidity. This was noticed when the pH was increased from negative alpha value (5.5) to positive alpha value (8.5) in which the residual turbidity, then, was found to increase from 224 NTU (run 1) to 232 NTU (run 10). Similarly, an increase in the coagulation time was discovered to lead to increase in residual turbidity from 192 NTU (run 3) to
197 NTU (run 12).
Within the ranges of the factors considered in the axial experiments, the coagulant dose was found to be the only factor that seriously affected the turbidity removal of the pumpkin seed coagulant. For instance,
comparing runs 18 and 4, it was observed that
increasing the coagulant dose from negative alpha value
to positive value resulted in decrease in residual turbidity from 320 NTU to 132 NTU. The maximum turbidity removal, in this case, when the pH was 7.00 and coagulation time was 7.5 min, was obtained to be approximately 71.73%.
Using the response obtained from the experiments carried out and given in Table 3, a model relating the residual turbidity of the synthesized wastewater to the factors (pH, coagulant dose and coagulation time) affecting the turbidity removal of the pumpkin seed coagulant that were considered was developed to be:
Y = 114.49 − 12.73 A + 117.33B + 5.53C − 13.08 AB + 7.63 AC − 6.72 BC − 4.86 A 2 − 3.23B 2 − 7.11C 2
(1)
The developed model, which was quadratic in
nature, was analysed, and the results of the analysis are as given in Table 4.![]()
Table 4: Results of analysis of surface response full quadratic model of the wastewater treatment process![]()
Source | Sum of squares | Degree of freedom | Mean square | F value | p-value | |
Model | 49993.82 | 9 | 5554.87 | 16.5 | < 0.0001 | significant |
A-pH | 1613.75 | 1 | 1613.75 | 4.79 | 0.0534 | |
B-Coagulant Dose | 37452.13 | 1 | 37452.13 | 111.22 | < 0.0001 | |
C-Coagulation Time | 137.98 | 1 | 137.98 | 0.41 | 0.5365 | |
AB | 1540.12 | 1 | 1540.12 | 4.57 | 0.0582 | |
AC | 4371.13 | 1 | 4371.13 | 12.98 | 0.0048 | |
BC | 1128.12 | 1 | 1128.12 | 3.35 | 0.0971 | |
A2 | 215.57 | 1 | 215.57 | 0.64 | 0.4422 | |
B2 | 301.61 | 1 | 301.61 | 0.9 | 0.3663 | |
C2 | 3557.53 | 1 | 3557.53 | 10.56 | 0.0087 | |
Residual | 3367.38 | 10 | 336.74 | |||
Lack of Fit | 3319.38 | 5 | 663.88 | 69.15 | 0.0001 | significant |
Pure Error | 48 | 5 | 9.6 | |||
Cor Total | 53361.2 | 19 | ||||
R-Squared = 0.9369; Adj R-Squared = 0.8801; Pred R-Squared = 0.4997 |
![]()
As it can be observed from the table, even
though the analysis of the model revealed that the entire
model, having a p-value of 0.0001, was significant with
R-squared, Adj R-squared and pred R-squared values of
0.9369, 0.8801 and 0.4997 respectively, it was discovered that some of the factors considered were insignificant because their p-values were greater than 5% (that is,
with 95% confidence level). Besides, the low value of the
pred R-squared of the model was an indication that the
model might not be able to predict the behaviour of the
process very well. As such, those insignificant factors,
except the coagulation time that was retained to avoid hierarchy problem of the model, were removed from the model so that it (the model) could be modified and
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1274
ISSN 2229-5518
improved. The modified model, which had its R-
squared, Adj R-squared and pred R-squared values of
0.9281, 0.8861 and 0.6693 respectively was obtained to
be:
Y = 394.97 − 80.80 A + 97.92 B + 0.36 C − 13.08 AB + 17.63 AC − 6.72 BC − 6.77C 2
(2)![]()
Table 5: Analysis of variance results for surface response modified quadratic model![]()
Source | Sum of squares | Degree of freedom | Mean square | F value | p-value | |
Model | 49522.63 | 7 | 7074.66 | 22.12 | < 0.0001 | significant |
A-pH | 1613.75 | 1 | 1613.75 | 5.04 | 0.0443 | |
B- Coagulant Dose | 37452.13 | 1 | 37452.13 | 117.08 | < 0.0001 | |
C-Coagulation Time | 137.98 | 1 | 137.98 | 0.43 | 0.5237 | |
AB | 1540.12 | 1 | 1540.12 | 4.81 | 0.0486 | |
AC | 4371.13 | 1 | 4371.13 | 13.66 | 0.0031 | |
BC | 1128.12 | 1 | 1128.12 | 3.53 | 0.0849 | |
C2 | 3279.41 | 1 | 3279.41 | 10.25 | 0.0076 | |
Residual | 3838.57 | 12 | 319.88 | |||
Lack of Fit | 3790.57 | 7 | 541.51 | 56.41 | 0.0002 | significant |
Pure Error | 48 | 5 | 9.6 | |||
Cor Total | 53361.2 | 19 | ||||
R-Squared= 0.9281; Adj R-Squared = 0.8861; Pred R-Squared = 0.6693; |
![]()
Desig n-Expert® Software
Turbidity
Color points by value of
Turbidity:
![]()
367
132
370.00
310.00
250.00
190.00
130.00

Predicted vs . Actual
132.00 190.75 249.50 308.25 367.00
Ac t ual
Figure 5: Predicted and experimentally measured residual turbidity of the synthesized dairy wastewater
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1275
ISSN 2229-5518
Desig n-Expert® Software
Turbidity
![]()
Desig n Points
![]()
367
132
X1 = A: pH
X2 = B: C.Dose
Actual Factor
C: Coag ulation Time = 7.50
4.189
3.595
3.000
2.405
1.811
Turbidity
211.716 233.797 6 255.878 277.959 300.04 | ||||
211.716 233.797 6 255.878 277.959 300.04 | ||||
211.716 233.797 6 255.878 277.959 300.04 | ||||
211.716 233.797 6 255.878 277.959 300.04 | ||||
6.11 6.55 7.00 7.45 7.89
A: pH
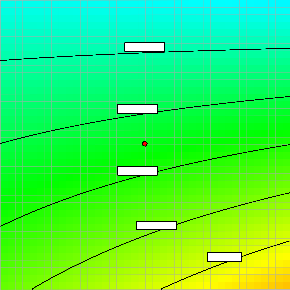
Figure 6. Effect of pH and coagulant dose on turbidity removal capacity of pumpkin seed coagulant
Moreover, the modified model was analysed
and its results of analysis, given in Table 5, have revealed that it (the model) was not only significant but can also perform well in prediction because an improvement has been recorded in its pred R-squared value. It has, thus, been discovered that the modified model has a better capability of predicting the behaviour of the process because its pred R-squared has increased from 0.4997 to 0.6693.
After obtaining the modified model that was
discovered to have good ability of predicting the behaviour of the process, it was simulated for prediction and the predicted residual turbidity values were plotted against the actual (experimental) values of the residual turbidity obtained from the treatment of the synthesized dairy wastewater using the developed pumpkin seed
coagulant, as shown in Figure 5. From the figure, it was
discovered that good correlations were found to exist between the predicted and the actual residual turbidity values.
Also investigated in this work was the interaction occurring between the factors considered to
be influencing the turbidity removal of the pumpkin seed coagulant from the wastewater. As a result of this, shown in Figure 6 is the interactive effect of pH and coagulant dose on the turbidity removal capacity of the pumpkin seed coagulant. According to the figure, increasing the coagulant dose while decreasing pH was found to lead to decrease in the residual turbidity of the wastewater, which indicated increase in the turbidity removal capacity of the coagulant.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1276
ISSN 2229-5518
Desig n-Expert® Software
Turbidity
![]()
Desig n Points
![]()
367
132
X1 = A: pH
X2 = C: Coag ulation Time
Actual Factor
B: C.Dose = 3.000
8.99
8.24
7.50
6.76
6.01

Turbidity
210.438 221.884 233.33 256.222 2464.776 233.33 221.884 | ||||
210.438 221.884 233.33 256.222 2464.776 233.33 221.884 | ||||
210.438 221.884 233.33 256.222 2464.776 233.33 221.884 | ||||
210.438 221.884 233.33 256.222 2464.776 233.33 221.884 | ||||
6.11 6.55 7.00 7.45 7.89
A: pH
Figure 7. The effect of pH and coagulation time on turbidity removal capacity of pumpkin seed coagulant
Also given in Figure 7 is the interactive effect of
pH and coagulation time on the turbidity removal
capacity of the pumpkin seed coagulant. From the
figure, it was noticed that, up to the centre point, a decrease in coagulation time with increase in pH was observed to lead to increase in residual turbidity. This means that the factors that are influencing the turbidity removal of the wastewater using the pumpkin seed coagulant must be regulated, especially, to optimum in order to achieve maximum efficiency of the treatment process.
Based on this, the modified model of the process
was optimized, and the results obtained from the optimization carried out, with the aid of Design Expert, showed that the minimum residual turbidity that could be achieved was 148.63 NTU when the optimum values of pH, coagulant dose and coagulation time were 6.11,
4.19 g/l and 8.99 min, respectively.
Now, comparing the results obtained using jar
experimental method and the response surface
methodology, it was found that the minimum value of
turbidity given by the jar experimental method was 135
NTU while that given by the response surface
methodology was 132 NTU. It, therefore, implied that
the response surface methodology employed in this
work was able to produce a better result than the
conventional jar experimental method of optimizing
turbidity removal capacity of coagulants.
From the results obtained, it has been discovered that the central composite design of the response surface methodology of the Design Expert has been successfully applied to optimizing the use of pumpkin seed coagulant for the treatment of dairy wastewater. It was also found that the turbidity removal of the developed coagulant was largely affected by coagulant dose. In addition, the results of the analysis of variance obtained showed that both the full and the reduced quadratic models of the process developed were significant with p-values less than 0.0001, even though the full quadratic model contained some insignificant factors and had low prediction capability. The results, thereafter, obtained from the simulation of the modified quadratic model of the process revealed that the developed model was very good and capable of predicting the behaviour of the process well because its R-Squared, Adj R-Squared and Pred R-Squared were estimated to be 0.9281, 0.8861 and 0.6693, respectively.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1277
ISSN 2229-5518
A pH
Adj Adjusted
B Coagulant dose (g/l)
C Coagulation time (min)
Pred Predicted
Y Residual turbidity (NTU)
The supports rendered by Mrs Sabo NdoGule of Civil Engineering Laboratory of Abubakar Tafawa Balewa University, Bauchi, Nigeria and Mr Suleiman Umar of Gubi Dam Water Treatment Plant Laboratory, Bauchi, Bauchi State, Nigeria towards the successful conduction of the experiments of this research work are highly acknowledged and appreciated.
REFERENCES
(1) Agatonovic-Kustrin, S., Zecevic, M., Zivanovic, L. and
Tucker, I.G. (1998). Application of Neural Networks for Response Surface Modeling in HPLC optimization, Analytica Chimica Acta, 364, 256-273.
(2) Ali, E.N. Muyibi, S.A. Salleh, H.N. Alam, M.Z. and Salleh, M.R.M. (2010). Production of Natural Coagulant from Moringa Oleifera Seed for the Application in Treatment of Low Turbidity Water. Journal of Water Resources and Protection, 2, 259-266.
(3) Alturkmani, A. (2006). Industrial Wastewater.
Environmental Consultant – Industrial City of Hassia – Homs Environmental Engineering Website Manager, Syria,
1-32.
(4) Baumes, L., Farrusseng, D., Lengliz, M. and Mirodatos, C. (2004). Using Artificial Neural Networks to Boost High- Throughput Discovery in Heterogeneous Catalysis. QSAR & Combinatorial Science, 29, 767-778.
(5) Birima, A.H. Hammad, H.A., Desa, M.N.M., and Muda, Z.C. (2013). Extraction of Natural Coagulant from Peanut Seeds for Treatment of Turbid Water. 4th International Conference on Energy and Environment, IOP Publishing, 1-4.
(6) Box, G.E.P. and Draper, N.R. (1987). Empirical Model
Building and Response Surfaces, Wiley, New Jersey, USA.
(7) Chi, G., Hu, S., Yang, Y. and Chen, T. (2012). Response Surface Methodology with Prediction Uncertainty: A Multi- Objective Optimisation Approach. Chemical Engineering Research and Design, 90, 1235-1244.
(8) Diaz, A. Rincon, N. Escorihuela, A. Fernandez, N. Chacin, E. and Forster, C.F. (1999). A Preliminary Evaluation of Turbidity Removal by Natural Coagulants Indigenous to Venezuela. Process Biochemistry, 35(3-4), 391-395.
(9) Dutta, J.R., Dutta, P.K. and Banerjee, R. (2004). Optimization of Culture Parameters for Extracellular Protease Production
from a Newly Isolated Pseudomonas sp. Using Response Surface and Artificial Neural Network Models. Process Biochemistry, 39, 2193-2198.
(10) Giwa, A. and Giwa, S.O. (2012). Optimization of Transesterification Reaction Integrated Distillation Column Using Design Expert and Excel Solver. International Journal of Advanced Scientific and Technical Research, 2(6), 423-435.
(11) Hadjmohammadi, M. and Kamel, K. (2008). Response Surface Methodology and Support Vector Machine for the Optimization of Separation in Linear Gradient Elution. Journal of Separation Science, 31, 3864-3870.
(12) http://www.whfoods.com/genpage.php?tname=foodspice&
dbid=82; Visiting Date: 22/02/2015.
(13) Myers, R.H. and Montgomery, D.C. (1995). Response
Surface Methodology, Wiley, New Jersey, USA.
(14) Ndabigengesere, A., Narasiah, K.S. and Talbot, B.G. (1995).
Active Agents and Mechanism of Coagulation of Turbid
Waters Using Moringa Oleifera. Water research, 29(2), 703-
710.
(15) Philips, R.J. (1997). State-of-the-Art Report: Food Manufacturing Coalition for Innovation and Technology Transfer. R. J. Philips & Associates, Inc., Virginia, 1-14.
(16) Reddy, V. (2014). Benefits of Pumpkin Seeds for Skin, Hair
and Health. Elephant journal. http://www.elephantjournal.com/2014/06/11- benefits-of-pumpkin-seeds-for-sk in-hair-health-vineetha- reddy/; Date visited: 22/02/2015.
(17) Shao, P., Jiang, S.T. and Ying, Y.J. (2007). Optimization of Molecular Distillation for Recovery of Tocopherol from Rapeseed Oil Deodorizer Distillate Using Response Surface and Artificial Neural Network Models. Food and Bioproducts Processing, 85, 85-92.
(18) Singh, U. and Kaushal, R.K. (2013). Treatment of Waste Water with Low Cost Adsorbent – A Review. VSRD International Journal of Technical & Non-Technical Research, 4(13), 33.
(19) Stat-Ease, Design Expert Version 7.0.0, Stat-Ease Inc., 2005. (20) Tang, Q., Lau, Y., Hu, S., Yan, W., Yang, Y. and Chen, T.
(2010). Response Surface Methodology Using Gaussian Processes: Towards Optimizing the Trans-Stilbene Epoxidation over Co2+–NaX Catalysts. Chemical Engineering Journal, 156, 423-431.
(21) Vara, S. (2012). Screening and Evaluation of Innate Coagulants for Water Treatment: A Sustainable Approach. International Journal of Energy and Environmental Engineering. 3:29, 1-11.
(22) Yan, W., Chen, Y., Yang, Y. and Chen, T. (2011a).
Development of High Performance Catalysts for CO Oxidation Using Data-Based Modeling. Catalysis Today,
174, 127-134.
(23) Yan, W., Hu, S., Yang, Y., Gao, F. and Chen, T. (2011b).
Bayesian Migration of Gaussian Process Regression for
Rapid Process Modeling and Optimization. Chemical
Engineering Journal, 166, 1095–1103.
IJSER © 2015 http://www.ijser.org