International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1430
ISSN 2229-5518
T. F. Adepoju1*, S.K. Layokun2, Ojediran, J. O3, Charles, O4
1Chemical Engineering Department, Landmark University, Omu-aran, P.M.B. 1001, Kwara State, Nigeria
2Chemical Engineering Department, Obafemi Awolowo University, Ile-Ife, Osun State, P.M.B, 2200055, Nigeria
3Agric&Biosystem Engineering Department, Landmark University, Omu-aran, P.M.B.1001, Kwara State, Nigeria
4Biological Sciences Department, Landmark University, Omu-aran, P.M.B. 1001, Kwara State, Nigeria
*Address of Corresponding Author: Chemical Engineering Department, Landmark University, Omu-aran, P.M.B. 1001, Kwara State, Nigeria.
![]()
………………………I………J………………S…………………E……………………R…………………………………..
Abstract: In this work, Response surface methodology (RSM) was employed to optimize the production of
benzyl alcohol (BA) via the biotransformation of benzaldehyde using free cell of Saccharomyces cerevisiae presence β -Cyclodextrin. Specifically, response surface methodology was applied, and the effect of five variables, viz. cell weight, incubation time, acetaldehyde conc., benzaldehyde conc. and β-CD level and their reciprocal were determined. Central composite rotatable design (CCRD) was used to generate 50 individual experiments, which were designed to study the effects of these factors during the process. A statistical model predicted the highest biotransformation yield of BA to be 327.259 (mg/100 ml) at the following ooptimized variables conditions: cell weight of 6.00 g (wet. wt), incubation time of 80 min, acetaldehyde conc. of 400.00 (µg/100 ml), benzaldehyde conc. of 500.00 (mg/100 ml) and β-CD level of 3.20 %. Using these variables under experimental condition in three independent replicates, an actual BA yield of 326.00 (mg/100 ml) was obtained. The physical properties of produced BA suggests that its could be used effectively in health care as well as in industries.
Index Terms: Biotransformation, Saccharomyces cerevisiae, optimization, Response surface methodology, benzyl alcohol (BA)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1431
ISSN 2229-5518
Benzyl alcohol (BA) also known as phenyl
methanol is an aromatic alcohol with the molecular formula C6 H5 CH2 OH. The benzyl group is regularly abbreviated "Bn" not to be confused with "Bz" which is used for benzoyl, hence BA general formula is denoted as BnOH. It’s a colorless liquid with a mild pleasant aromatic odour. Meanwhile, it’s partially soluble in water and completely miscible in alcohols and diethyl ether. It is a widely used organic solvent due to its
polarity, low toxicity, and low vapor pressure.
BA is used as a
general solvent for inks, paints, lacquers,
and epoxy resin coatings, Furuta et al. (1995). It is also a precursor to a variety of esters, used in the soap, perfume, and flavor industries. It is often added to intravenous medication solutions as a preservative due to its bacteriostatic and antipruritic properties. It is also used as a photographic developer, used as a dielectric solvent for the
dielectrophoretic reconfiguration of nanowires![]()
IJSE(Wissner-GrosRs, 2006). It is oxidized rapidly in
• Adepoju T.F is currently pursuing
master degree program in Chemical
Engineering at Obafemi Awolowo
University, Nigeria. He is a Lecturer in
the department of Chemical Engineering, Landmark University, Email: avogadros2002@yah.com
• S. K. Layokun is a professor in the Department of Chemical Engineering, Obafemi Awolowo University, Nigeria.
• Ojediran O.J is a senior Lecturer from the Agric & Biosystem Engineering Department, Landmark University
• C. Okolie is Lecturer in biological Sciences Department, Landmark University, Nigeria![]()
:
healthy individuals to benzoic acid, conjugated
with glycine in the liver, and excreted as hippuric acid. Although, high concentrations can result in toxic effects including respiratory failure, vasodilation, hypotension, convulsions, and paralysis. Newborns, especially if critically ill, may not metabolize BA as readily as adults.
U.S. FDA approved 5% solution usage of BA in the treatment of head lice in children older than 6 months and in adults (Sciele Pharmaceuticals, Inc., 2009). Benzyl alcohol lotion is used to treat head lice (small insects that attach
themselves to the skin) in adults and children 6
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1432
ISSN 2229-5518
months of age and older. But it’s not advisable to
be used in children less than 6 months of age. BA has nearly the same refraction index of quartz and wool fibre. If a clear quartz object is immersed in BA, it becomes almost invisible. This has been used as a method to non- destructively recognize if an object is made of true quartz or not. Similarly, white wool immersed in BA also becomes almost invisible clearly revealing contaminants such as dark and medullated
fibres and vegetable matter.
Studies revealed that the formation of BA from
benzaldehyde under normal fermentative conditions using yeast, shows that the quantitative conversion has never been achieved because of formation of by-products like L-PAC, PAC-diol (Smith and Hendlin, 1953; Gupta et al., 1979; Netraval and Vojtisek, 1982; Agrawal and Basu,
1989). The yeast cannot be used for multiple batches because of the toxic and inhibitory effects of substrate and products (Long et al., 1989;
Coughlin et al., 1991). Although, the use of
IJSER
BA is produced naturally by many plants
and is commonly found in fruits and teas. It is also found in a variety of essential oils including jasmine, hyacinth, and ylang-ylang (Merck ECDB, 1989). It is also one of the chemical compounds found in castoreum. This compound is gathered from the beaver plant food (Muller- Schwarze, 2003). It is also a bi-production in biotransformation of benzyladehyde to Phenylacetylcarbinol (L-PAC). Meanwhile, almost all the literature concerning the synthesis of L-PAC and benzyl alcohol by fermenting yeast deals with yield optimization by free cells (Agrawal et al.,
1986; Cardillo et al., 1991; Zeeman et al., 1992).
cyclodextrin always decreased the toxicity of
benzaldehyde for bioconversion using immobilized cells has been reported (Coughlin et al., 1991; Mahmoud et al., 1990).
Response Surface Methodology (RSM), a useful optimization tool has been applied in research to study the effect of individual variables and their interactions on response variables. It has been used extensively in the optimization processes. (Mohammed et al., 2008; Mitra et al.,
2009; Njoku et al., 2009; Tan et al., 2009). The main advantage of RSM is the ability to reduced number of experimental runs needed to provide sufficient
information for statistically acceptable results. In
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1433
ISSN 2229-5518
view of these, this work dwells on optimization of
production of BA via the biotransformation of benzaldehyde to L-PAC using the free cells of Saccharomyces cerevisiae. To optimize the biotransformation conditions, RSM was applied to determine the effects of five -level-five factors and their reciprocal interactions on the yield of BA.
2.1 Materials
All the chemicals (diethyl ether, anhydrous sodium sulphate, benzaldehyde, acetyladehyde, β-
Suspension of cells of the isolate Saccharomyces
cerevisiae (1.0 ml) containing 106 cells was inoculated into 9 ml of growth medium and incubated on a rotary shaker at 30 ± 2oC at 240 rpm for 24 h. The obtained culture was inoculated into
100 ml of the same medium and allowed to grow for 24 h. Under the same conditions, cells were harvested by centrifuging at 10, 000 rpm for 15 min at 15 oC. The biomass obtained was washed with water, centrifuged and was used for
biotransformation studies.
IJSER
cyclodextrin ((β- CD) etc.) used were of analytical
grade and need no further purification.
2.2 Methods
2.2.1 Microorganisms
Saccharomyces cerevisiae used in this study was isolated locally. The culture was consistently maintained on a medium containing 0.4%
dextrose, 1% yeast extract, 1% malt extract, and 2%
agar at pH 7.2 ( Agarwal et al., 1986).
2.2.2 The growth medium
The growth medium for Saccharomyces cerevisiae
(Long et al., 1989) contained glucose 2%, peptone
2%, yeast extract 1% and had pH 5.5.
2.2.3 Culture growth
2.2.4 Biotransformation of benzaldehyde
Biotransformation medium (100 ml)
containing 5% glucose, 0.6% peptone and had pH
4.5 was inoculated with a known weight of cell mass (biomass) obtained. The reactor was incubated on a shaker at 30 oC and 240 rpm at different time range for adaptation of cells to the medium. Benzaldehyde and acetaldehyde was added and flasks were incubated again for the biotransformation on a shaker at 30 oC and 240 rpm.
2.2.5 Effect of b-cyclodextrin addition on biotransformation of benzaldehyde
Effect of 0.4 – 3.2% β-cyclodextrin (β-CD)
was studied at benzaldehyde and acetaldehyde
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1434
ISSN 2229-5518
levels ranging from 500 mg to 1600 mg/100 ml and
400 µl to 1300 µl/100 ml, respectively. The reaction was allowed to take place for 3 h at 30 ± 2oC and
240 rpm. Semi-continuous feeding of different
levels of benzaldehyde and acetaldehyde was also carried out according to design software (Table 1) at different intervals in presence of β-CD.
2.3 Analysis of biotransformation products
After biotransformation, the medium was centrifuged at 10,000 rpm for 15 min. The supernatant were extracted three times with equal
250 oC and holding time was for 5 min. Retention
times of BA (benzyl alcohol) was 13 min. The concentration of the compound was determined using peak area method (Shukla and Kulkarni,
1999). The experiment was replicated in triplicate until it was found to be reproducible within ± 3 percent limits.
2.5 Experimental design
Central Composite Rotatable Design (CCRD) experimental design was employed to optimize the biotransformation process. Five-level-
IJSER
volumes of diethylether. The combined extract was
dried over anhydrous sodium sulphate and concentrated over a temperature controlled water bath. The residue obtained was dissolved in methanol and subjected to gas chromatography (GC) analysis.
2.4 Gas Chromatography Analysis
The conditions used for GC analysis were as follows- GC model used was Chemito-8510 with Oracle -1 computing integrator. A 4 meter long column of 5% OV-17 was used. The injector temperature and detector temperature (FID) was maintained at 250 oC. Column programming was
as follows: 75 oC for 3 min, then 10 oC/ 1 min up to
five-factors design was applied, which generate 50
experimental runs. This included 32 factorial points, 10 axial points, and 8 central points to provide information regarding the interior of the experimental region, making it possible to evaluate the curvature effect. Selected factors for biotransformation processes were; cell weight g (wet. wt.): X1 , incubation time (min): X2 , Acetaldehyde conc. (mg/100 ml): X3 , benzaldehyde conc. (mg/100 ml): X4 and β-CD level (%): X5 . Table
1 show the independent factors and their five levels for Central Composite design, and the combinations of five independent factors in a
Central Composite experimental design.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1435
ISSN 2229-5518
![]()
Depicted in Table 2 also are the
experimentally obtained benzyl alcohol yields, the predicted yields and the residual values. Figure 1 showed the plot of actual against the predicted yield. The effects of unexplained variability in benzyl alcohol yield response due to extraneous factors were minimized by randomizing the order of experiments.
9 -1 -1 -1 1 -1 273.00 273.15 -0.15
10 1 -1 -1 1 -1 294.00 294.23 -0.23
11 -1 1 -1 1 -1 304.00 303.53 0.47
12 1 1 -1 1 -1 305.00 304.98 0.024
13 -1 -1 1 1 -1 313.00 313.25 -0.25
14 1 -1 1 1 -1 319.00 318.70 0.30
15 -1 1 1 1 -1 331.00 331.24 -0.24
16 1 1 1 1 -1 317.00 317.07 -0.069
17 -1 -1 -1 -1 1 305.00 305.43 -0.43
18 1 -1 -1 -1 1 321.00 320.51 0.49
19 -1 1 -1 -1 1 332.00 331.81 0.19
20 1 1 -1 -1 1 327.00 327.26 -0.26
21 -1 -1 1 -1 1 300.00 300.03 -0.028
22 1 -1 1 -1 1 299.00 299.48 -0.48![]()
Variab le
Sym![]()
bol Coded factor levels
-2 -1 0 1 2
23 -1 1 1 -1 1 314.00 314.03 -0.026
24 1 1 1 -1 1 294.00 293.85 0.15
25 -1 -1 -1 1 1 282.00 281.93 0.067![]()
IJSER
CW X1 2 3 4 5 6
IT X2 40 50 60 70 80
AC X3 400 700 1000 1300 1600
BC X4 500 700 900 1100 1300
26 1 -1 -1 1 1 300.00 300.13 -0.13
27 -1 1 -1 1 1 297.00 297.18 -0.18
28 1 1 -1 1 1 296.00 295.76 0.24
ß-CD level (%)
X5 0.4 0.8 1.2 1.6 3.2
29 -1 -1 1 1 1 266.00 265.90 0.099
30 1 -1 1 1 1 268.00 268.48 -0.48
31 -1 1 1 1 1 269.00 268.77 0.23
CW= Cell weight g (wet. wt), IT= Incubation time (min), AC= Acetaldehyde conc. (µg/100 ml), BC= Benzaldehyde conc. (mg/100 ml)
32 1 1 1 1 1 251.00 251.73 -0.73
33 -2 0 0 0 0 300.00 300.37 -0.37
34 2 0 0 0 0 302.00 301.44 0.56
35 0 -2 0 0 0 337.00 336.69 0.31
36 0 2 0 0 0 366.00 366.12 -0.12![]()
37 0 0 -2 0 0 297.00 297.43 -0.43
38 0 0 2 0 0 306.00 305.38 0.62![]()
39 0 0 0 -2 0 320.00 320.11 -0.11
40 0 0 0 2 0 288.00 287.70 0.30
41 0 0 0 0 -2 266.00 266.22 -0.22
42 0 0 0 0 2 245.00 244.59 0.41
43 0 0 0 0 0 277.00 277.48 -0.48
44 0 0 0 0 0 278.00 277.48 0.52
45 0 0 0 0 0 277.00 277.48 -0.48
46 0 0 0 0 0 278.00 277.48 0.52![]()
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1436
ISSN 2229-5518
![]()
47 0 0 0 0 0 277.00 277.48 -0.48
48 0 0 0 0 0 278.00 277.48 0.52
49 0 0 0 0 0 277.00 277.48 -0.48
50 0 0 0 0 0 278.00 277.48 0.52
The fitted quadratic response model is described
by Eq. 1:
𝑘 𝑘 𝑘
2
𝑌 = 𝑏0 + � 𝑏𝑖 𝑋𝑖 + � 𝑏𝑖𝑖 𝑋𝑖 + � 𝑏𝑖𝑗 𝑋𝑖 𝑋𝑗 + 𝑒 (1)
PV=predicted value (mg/100 ml), Res. = Residual,
SR= Standard Runs
𝑖 =1
𝑖 =1
𝑖 <𝑗
Where: Y is BA yield (response factor), bo is the
380.00
360.00
340.00
320.00
Predicted vs. Actual
intercept value, bi (i= 1, 2,……… k) is the first order model coefficient, bij is the interaction effect, and
bii represents the quadratic coefficients of Xi, and e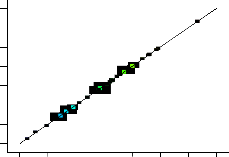
300.00
280.00 44
260.00
240.00
240.00 260.00 280.00 300.00 320.00 340.00 360.00 380.00
Actual
Table 2 shows the coded factors
IJSEconsidered inRthis study with BA yield, predicted
2.5.1 Statistical Data Analysis
The data obtained from biotransformation process to produce BA (benzyl alcohol) was analysed statistically using response surface methodology (CCRD), so as to fit the quadratic polynomial equation generated by the Design- Expert software version 8.0.3.1 (Stat-Ease Inc., Minneapolis, USA). To correlate the response variable to the independent variables, multiple regressions was used to fit the coefficient of the polynomial model of the response. The quality of the fit of the model was evaluated using test of
significance and analysis of variance (ANOVA).
value as well as the residual values obtained. Design Expert 8.0.3.1 software was employed to evaluate and determine the coefficients of the full regression model equation and their statistical significance. Table 3 described the results of test of significance for every regression coefficient. Considering the test for comparing the variance associated with all terms with the residual variance (large F-values) and low corresponding probability value that is associated with the F -value for all terms (p-values), all the model terms are remarkably significant and have very strong effects
on the BA yield with p< 0.05 (Table 3).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1437
ISSN 2229-5518
Nevertheless, the linear term 𝑋 2
with F-
adequate representation of the actual relationship
value of 42611.15 and p-value <0.0001, is the most
significant model term. In order to minimize error, all the coefficients were considered in the design. The results of the second-order response surface model fitting in the form of ANOVA are presented in Table 4. The model F-value (terms used to estimate effects) of 29283.62 with low p-value (<0.0001) implied a high significance for the regression model (Yuan et al., 2008). The goodness
of fit of the model was checked by the coefficient of
among the selected factors. The lack-of-fit term of
0.7490 was not significant relative to the pure error. In this case, a non-significant lack of fit is![]()
![]()
determination (R2). R2 should be at least 0.80 for the good fit of a model (Guan and Yao, 2008). In this case, the R2 value of 0.9998 indicated that the sample variation of 99.98% for the BA production is attributed to the independent factors (cell
weight, incubation time, acetaldehyde conc.,
benzaldehyde conc. and β-CD level) only 0.02% of
the total variations were not explained by the model. The value of the adjusted determination coefficient (Adj. R2 of 0.996) was also very high, supporting a high significance of the model (Akhnazarova and Kefarov, 1982; Khuri and Cornell, 1987) and all p-values were less than 0.05,
implying that the model proved suitable for the
2
![]()
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1438
ISSN 2229-5518
𝑥3 𝑥5 𝑎𝑛𝑑 𝑥4𝑥5) and the quadratic (𝑥2 ) have
negative impact on BA yield, whereas, the rest of
the products have positive effect on the yield. The model coefficients and probability values i.e. coded value are shown in Table 5. The low values of standard error observed in the intercept and all the
model terms showed that the regression model fits
SS= Sum of Square, MS= mean square
good. Hence, the model could be used in theoretical prediction of the BA production. The
developed regression model equation describing
the data well, and the prediction is good (Table 5).
The variance inflation factor (VIF) obtained in this study showed that the 8-centre points are orthogonal to all other factors in the model. The
IJSEmodel also Rproved suitable for the adequate
the relationship between the BA yield (Y) and the
coded values of independent factors of cell weight (X1 ), incubation time (X2 ), acetaldehyde conc. (X3 ), benzaldehyde (X4 ) and β-CD level (X5 ) and their respective interactions is described in Eq. (2).
𝑌2 = 277.48 + 0.23𝑥1 + 6.19𝑥2 + 1.67𝑥3 − 6.81𝑥4
− 4.55𝑥5 − 4.91𝑥1 𝑥2 − 3.91𝑥1 𝑥3
+ 0.78𝑥1 𝑥4 − 0.72𝑥1 𝑥5
− 3.09𝑥2 𝑥3−2.78𝑥2𝑥4 − 3.78𝑥2𝑥5
− 2.66𝑥3 𝑥4 − 14.03𝑥3 𝑥5
representation of the real relationship among the selected independent factors.
Usually, the three-dimensional (3D) response surface plots are graphical representations of the regression equation for the optimization of the reaction variables, and they are represented in
2 2 Table 5: Regression coefficients and significance
− 9.59𝑥4 𝑥5 + 4.14𝑥1 + 13.07𝑥2
2 2 of response surface quadratic
+ 4.23𝑥3 + 4.67𝑥4
− 3.90𝑥2![]()
(2)![]()
Where 𝑌2 = BA yield (mg/100 ml)
The linear (𝑥3 , 𝑥4 , 𝑥5 ), all the cross
products (𝑥1 𝑥2 , 𝑥1 𝑥3, 𝑥1 𝑥5 , 𝑥2 𝑥3, 𝑥2 𝑥4, 𝑥2 𝑥5 , 𝑥3 𝑥4,
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1439
ISSN 2229-5518
benzaldehyde conc., cell weight with β-CD level,
X3 | 1.67 | 1 | 0.072 | 1.53 | 1.82 | 1.00 | acetaldehyde conc. with benzaldehyde conc., and acetaldehyde conc. with β-CD level, respectively. The optimum values of the independent factors selected for the biotransformation of benzaldehyde to BA were obtained by solving the regression equation (Eq. 2) using the Design- Expert software package. The optimum conditions for this process were statistically predicted as X1 = |
X4 | -6.81 | 1 | 0.072 | -6.96 | -6.67 | 1.00 | acetaldehyde conc. with benzaldehyde conc., and acetaldehyde conc. with β-CD level, respectively. The optimum values of the independent factors selected for the biotransformation of benzaldehyde to BA were obtained by solving the regression equation (Eq. 2) using the Design- Expert software package. The optimum conditions for this process were statistically predicted as X1 = |
X5 | -.4.55 | 1 | 0.072 | -4.69 | -4.40 | 1.00 | acetaldehyde conc. with benzaldehyde conc., and acetaldehyde conc. with β-CD level, respectively. The optimum values of the independent factors selected for the biotransformation of benzaldehyde to BA were obtained by solving the regression equation (Eq. 2) using the Design- Expert software package. The optimum conditions for this process were statistically predicted as X1 = |
X1 X2 | -4.91 | 1 | 0.083 | -5.08 | -4.74 | 1.00 | acetaldehyde conc. with benzaldehyde conc., and acetaldehyde conc. with β-CD level, respectively. The optimum values of the independent factors selected for the biotransformation of benzaldehyde to BA were obtained by solving the regression equation (Eq. 2) using the Design- Expert software package. The optimum conditions for this process were statistically predicted as X1 = |
X1 X3 | -3.91 | 1 | 0.083 | -4.08 | -3.74 | 1.00 | acetaldehyde conc. with benzaldehyde conc., and acetaldehyde conc. with β-CD level, respectively. The optimum values of the independent factors selected for the biotransformation of benzaldehyde to BA were obtained by solving the regression equation (Eq. 2) using the Design- Expert software package. The optimum conditions for this process were statistically predicted as X1 = |
X1 X4 | 0.78 | 1 | 0.083 | 0.61 | 0.95 | 1.00 | acetaldehyde conc. with benzaldehyde conc., and acetaldehyde conc. with β-CD level, respectively. The optimum values of the independent factors selected for the biotransformation of benzaldehyde to BA were obtained by solving the regression equation (Eq. 2) using the Design- Expert software package. The optimum conditions for this process were statistically predicted as X1 = |
X1 X5 | -0.72 | 1 | 0.083 | -0.89 | -0.55 | 1.00 | acetaldehyde conc. with benzaldehyde conc., and acetaldehyde conc. with β-CD level, respectively. The optimum values of the independent factors selected for the biotransformation of benzaldehyde to BA were obtained by solving the regression equation (Eq. 2) using the Design- Expert software package. The optimum conditions for this process were statistically predicted as X1 = |
X2 X3 | -3.09 | 1 | 0.083 | -3.26 | -2.92 | 1.00 | acetaldehyde conc. with benzaldehyde conc., and acetaldehyde conc. with β-CD level, respectively. The optimum values of the independent factors selected for the biotransformation of benzaldehyde to BA were obtained by solving the regression equation (Eq. 2) using the Design- Expert software package. The optimum conditions for this process were statistically predicted as X1 = |
X2 X4 | -2.78 | 1 | 0.083 | -2.95 | -2.61 | 1.00 | acetaldehyde conc. with benzaldehyde conc., and acetaldehyde conc. with β-CD level, respectively. The optimum values of the independent factors selected for the biotransformation of benzaldehyde to BA were obtained by solving the regression equation (Eq. 2) using the Design- Expert software package. The optimum conditions for this process were statistically predicted as X1 = |
X2 X5 | -3.78 | 1 | 0.083 | -3.95 | -3.61 | 1.00 | acetaldehyde conc. with benzaldehyde conc., and acetaldehyde conc. with β-CD level, respectively. The optimum values of the independent factors selected for the biotransformation of benzaldehyde to BA were obtained by solving the regression equation (Eq. 2) using the Design- Expert software package. The optimum conditions for this process were statistically predicted as X1 = |
X3 X4 | -2.66 | 1 | 0.083 | -2.83 | -2.49 | 1.00 | acetaldehyde conc. with benzaldehyde conc., and acetaldehyde conc. with β-CD level, respectively. The optimum values of the independent factors selected for the biotransformation of benzaldehyde to BA were obtained by solving the regression equation (Eq. 2) using the Design- Expert software package. The optimum conditions for this process were statistically predicted as X1 = |
X3 X5 | -14.03 | 1 | 0.083 | -14.20 | -13.86 | 1.00 | acetaldehyde conc. with benzaldehyde conc., and acetaldehyde conc. with β-CD level, respectively. The optimum values of the independent factors selected for the biotransformation of benzaldehyde to BA were obtained by solving the regression equation (Eq. 2) using the Design- Expert software package. The optimum conditions for this process were statistically predicted as X1 = |
X4 X5 | -9.59 | 1 | 0.083 | -9.76 | -9.42 | 1.00 | acetaldehyde conc. with benzaldehyde conc., and acetaldehyde conc. with β-CD level, respectively. The optimum values of the independent factors selected for the biotransformation of benzaldehyde to BA were obtained by solving the regression equation (Eq. 2) using the Design- Expert software package. The optimum conditions for this process were statistically predicted as X1 = |
2 X1 | 4.14 | 1 | 0.063 | 4.01 | 4.27 | 1.05 | 6.0 g (wet. wt.), X2 = 80 (min), X3 = 400.00 (µg/100 |
2 X2 | 13.07 | 1 | 0.063 | 12.94 | 13.20 | 1.05 | ml), X4 = 500 (mg/100 ml) and X5 = 3.20 %. The |
IJSER
X 2 4.23 1 0.063 4.10 4.36 1.05
X 2 4.67 1 0.063 4.54 4.80 1.05
X 2 -3.90 1 0.063 -4.03 -3.77 1.05
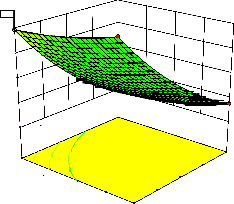
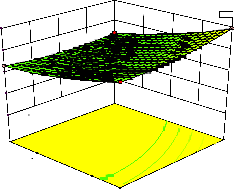
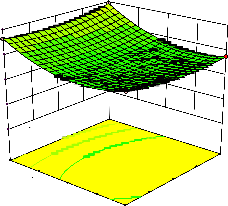
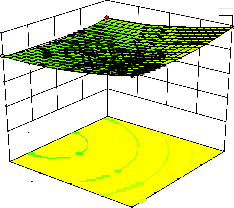
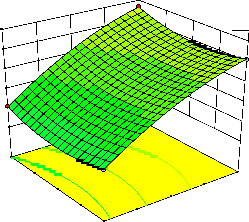
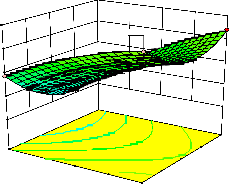
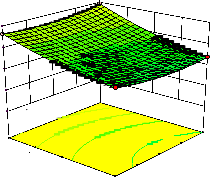
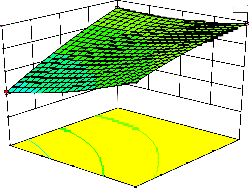
Figure 2. The curvatures’ nature of 3D surfaces in Figure 2a, b, e, f, g, and i suggested reciprocal interaction of cell weight with incubation time, cell weight with acetaldehyde conc., incubation time with acetaldehyde conc., incubation time with
benzaldehyde conc. incubation time with β-CD
predicted BA yield under the above set conditions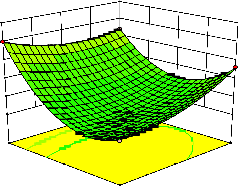
was 327.259 (mg/100 ml). In order to verify the prediction of the model, the optimum conditions were applied to three independent replicates, and the average BA yield obtained was 326.00 (mg/100 ml), which was well within the range predicted by the model equation.
340
332
324
316
level and benzaldehyde conc. with β-CD level, respectively. Meanwhile, the nature of curvatures’
of 3D surfaces in Figure 2c, d, h, j showed
308
300
1.00
0.50
0.00
0.00
0.50
1.00
moderate interactions of cell weight with
Incubationtime(min)
-0.50
-1.00
-1.00
-0.50 Cell weight (wet.wt)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1440
ISSN 2229-5518
(a)
(d)
338.12
338.12
340
340
315
315
290
290
265
265
240
240
-1.00
-0.50
0.00
0.00
-0.50
-1.00
1.00
0.50
0.00
0.00
-0.50
-1.00
taldehydeconc. (µg/100ml)
0.50
1.00
1.00
0.50
Cell weight (wet.wt)
Incubationtime(min)
-0.50
-1.00
1.00
Acet0.a5l0dehydeconc. (µg/100ml)
(b)
(e)
340
338.12
340
315
290
315
IJSE290 R
265
265
240
240
1.00
0.50
0.00
-0.50
-1.00
1.00
0.50
0.00
0.00
-0.50
-1.00
Cell weight (wet.wt)
0.00
-0.50
-1.00
(c)
Benza0.l5d0ehydeconc. (mg/100ml)
1.00
Incubationtime(min)
-0.50
-1.00
(f)
Benz0a.5ld0ehydeconc. (mg/100ml)
1.00
335
327.259
330
327.259
326
315
317
300
308
299
285
290
270
-1.00
-1.00
-0.50
0.00
0.00
-0.50
-1.00
-0.50
Cell weight (wet.wt)
0.00
0.50
-0.50
0.00
0.50
1.00
ß-CDlevel (%)
0.50
1.00
(g)
1.00
0.50 Incubationtime(min)
1.00
-1.00
ß-CDlevel (%)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1441
ISSN 2229-5518
340
315
290
265
240
In other to ascertain the quality of the L-
PAC produced, the physical properties was carried out, the appearance was found to be colourless
liquid with density 1.03 kg/dm3, the boiling points
1.00
0.50
0.00
0.00
-0.50
-1.00
was found to be 204 ± 2 0C and its partially soluble
cetaldehydeconc. (µg/100ml)
-0.50
-1.00
Benz0a.5ld0ehydeconc. (mg/100ml)
1.00
in water.
(h)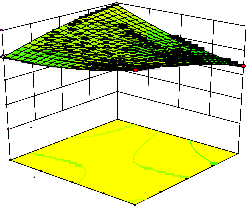
340
315
0 290
265
240
The results obtained in this study using response surface methodology to determine the
IJSEeffects of fivRe reaction variables, namely, cell
1.00
0.50
0.00
0.00
-0.50
-1.00
weight, incubation time, acetaldehyde conc.,
cetaldehydeconc. (µg/100ml)
340
315
290
265
240
-0.50
(i)
-1.00
1.00
0.50
ß-CDlevel (%)
338.12
benzaldehyde conc. and β-CD level on biotransformation of benzaldehyde via free cell Saccharomyces cerevisiae presence of Beta- Cyclodetrin, indicate that the BA produced was high. The statistical model predicted the optimal
conditions for the selected biotransformation
1.00
0.50
0.00
0.00
-0.50
-1.00
variables as cell weight of 6.00 g (wet. wt),
nzaldehydeconc. (mg/100ml)
-0.50
-1.00
(j)
1.00
0.50
ß-CDlevel (%)
incubation time of 80 min, acetaldehyde conc. of
400.00 (µl/100 ml), benzaldehyde conc. of 500.00
(ml/100 ml) and β-CD level of 3.20 % with an actual BA yield of 326.00 (mg/100 ml). Hence, this work established the usefulness of RSM for the
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1442
ISSN 2229-5518
optimum biotransformation of benzaldehyde to
BA and also the quality of BA produced advocated that it could be used effectively in health care as well as other industrial applications.
The Authors acknowledge the effort of the technical staff of biology and chemistry laboratories of Landmark University. The effort of Chief Technology of ABU, Zaria is highly appreciated.
1. Agarwal S.C., Basu, S.K., Vora, V.C,
Cyclohexene-1-Carboxaldehyde", Org.
Synth. 72: 86, Vol. 9: 722.
4. Coughlin, R.W., Mahmoud, W.M. and El- sayed, A.H. (1991): A.H. (1991). Enhanced bioconversion of toxic substances. US Patent. 5173-5413.
5. Guan, X., Yao, H., (2008): Optimization of viscozyme L-assisted extraction of oat bran protein using response surface methodology. Food Chemistry. 106: 345–
351.
IJSER
Mason, J.R. Pirt, S.J. (1986): Studies on the
production of Acetyl Phenyl Carbinol by yeast Employing Benzaldehyde as Precursor. Biotechol. Bioeng., 29(6), 783-785.
2. Cardillo, R., Servi S., Tinti, C. (1991): Biotransformation of unsaturated aldehydes by micro-organisms with pyruvate decarboxylase activity. Applied Microbiol. Biotechnol., 36(3), 300-303.
3. Furuta, K. Gao, Qing-Zhi, Yamamoto, Hisashi. (1995): "Chiral (Acyloxy)borane Complex-Catalyzed Asymmetric Diels- Alder Reaction: (1R)-1,3,4-Trimethyl-3-
6. Gupta, K.G. Singh, J., Sahani, G. and
Dhavan, S. (1979): Production of phenyl acetyl carbinol by yeasts. Biotechnol. Bioeng., 21(6), 1085-1089.
7. Khuri, A.I., Cornell, J.A., (1987): Response surfaces: design and analysis. New York: Marcel Dekker.
8. Long, A., James, P. and Ward, O.P. (1989):
Aromatic aldehydes as substrate for yeast and yeast alcohol dehydrogenase. Biotechnol. Bioeng., 33(5), 657-660.
9. Mahmoud, W. M., El-Sayed, A.H.M. and
Coughlin, R.W. (1990): Effect of β – Cyclodetrine on production of L-phenyl
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1443
ISSN 2229-5518
acetyl carbinol by immobilized cells of
Saccharomyces cerevisiae. Biotechnol. Bioeng.,
36(3), 256-262.
10. Mitra, P., Ramaswamy, H.S., Chang, K.S. (2009): Pumpkin (Cucurbita maxima) seed oil extraction using supercritical carbon dioxide and physicochemical properties of the oil. Journal of Food Engineering, 95, 208-213.
11. Mohammed, M.I., Hamza, Z.U., (2008):
Physicochemical properties of oil extracts
International Journal of Applied Resource and
Natural Product, 2(4):11-19.
15. Prescribing Information for Ulesfia Lotion, Sciele Pharmaceuticals, Inc., Sept. 2013, retrieved. 2013-09-28.
16. Tan, C.H., Ghazali, H.M., Kuntom, A., Tan, C.P., Ariffin, A.A. (2009): Extraction and physicochemical properties of low free fatty acid crude palm oil. Food Chemistry, 113: 645–650.
17. Smith, P. F. and Hendlin, D. (1953):
from
IJSEMechRanism of phenyl acetyl carbinol
Sesamum indicum L. seeds grown in Jigawa
State-Nigeria.Journal of Applied Science and Environmental Management, 12(2):99-
101.
12. Muller-Schwarze, D. (2003): The Beaver: Its Life and Impact. Pp: 43
13. Netraval, J. and Vojtisek, V. (1982):
Production of Phenylacetylcarbinol in various yeast species. Eur. J. Appl. Microbiol. Biotechnol., 16: 35-38.
14. Njoku, O.U., Boniface, J.A.E., Obitte, N.C., Odimegwu, D.C., Ogbu, H.I. (2009): Some
nutriceutical potential of beniseed oil.
synthesid by yeast. J. Bacteriol., 65, 440-
445.
18. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals –ECDB- (11th ed.), Merck, 1989, ISBN 091191028X,
1138.
19. Wissner-Gross, A. D. (2006): "Dielectrophoretic reconfiguration of nanowire
interconnects", Nanotechnology, 17, 4986-
499.
20. Zeeman, R., Netral, J., Bulantova, H., Vodnasky, M. (1992): Biosynthesis of
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1444
ISSN 2229-5518
phenyl acetyl carbinol in yeast
saccharomyces cerevisiae fermentation.
Pharmazie, 47(4), 291-294.
IJSER
IJSER © 2013 http://www.ijser.org