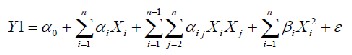International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1863
ISSN 2229-5518
Optimization for autolysis assisted production of fish protein hydrolysate from underutilized fish Pellona ditchela
Prabha.J*., Akhila Narikimelli., Maria Infanshia Sajini., S.Vincent.
Centre for Environmental Research and Development Department of Advanced Zoology and Biotechnology Loyola College, Chennai, India.
Abstract—Response surface methodology (RSM), based on Central composite design was used to optimize the hydrolyzing conditions, for the preparation of soluble protein hydrolysate from underutilized fish Pellona ditchela. Most favorable interfacing external factors, such as time, tempera- ture and pH on Degree of hydrolysis were determined from the model equations of RSM. Hydrolyzing time of 90min, pH 5 and temperature of 50oC was found to be the ideal condition to attain higher DH of 40.2% in acidic condition. Proximate analysis revealed that protein hydrolysate had relatively high protein (74.0 ± 0.2 %) and low lipid (0.77±0.1%) content. The chemical score of the hydrolysate indicates that it fulfils adult human nutritional require- ments. The amino acid composition of the protein hydrolysate verified to have the potential for application as an ingredient for poultry diet and adult human requirement. Protein hydrolysate from Pellona ditchela prepared, involving mild inorganic acid is cost effective and eco-friendly approach and the product obtained can potentially serve as a good source of desirable peptides and amino acids.
Index Terms— Pellona ditchela, Hydrolysate, Degree of hydrolysis, RSM, Chemical score.
—————————— ——————————
1 INTRODUCTION
IJSER
Exploitation of aquatic environment is major cause for the destruction of the fish population. FAO (2010) reports es- timates that by 2020 about 38% of total fish produced expected to export. As the fish production is increasing each year, the discarding rate also increases. About 38.5 million tones of spe- cies, globally discarded as by-catch due to low economic val- ue. These unusable or unwanted sub-sets off discards, known as ‘by-catch’, is subsequently thrown back to the sea in dead or dying condition (Harrington et al., 2006). Issue due to by- catch has major adverse ecological impacts on ocean conserva- tion and resource management of fisheries. (Lewison et al.,
2004).
Pellona ditchela is known as Pellona ditchelee, is a spe- cies of long finned herring native to the coast mangrove, swamps and estuaries of Indian ocean and Western Pacific generally in tropical waters. Pellona ditchela is reported as a common bycatch (Zymudheen et al., 2004) mostly seen in In- do-West Pacific South-Africa. It feeds on small planktonic or- ganisms especially diatoms (Siddique et al., 2007). Pellona ditchela referred to a non-target species and commercial dis- cards with less consumer preference and low economic value. Pellona ditchela noted to be major discards of the catch, which leads to threaten factor creating environmental issues. Dis- cards reduction is a key to any transition to a sustainable and resilient fishery and a keystone of the ecosystem approach. Novel processing methods needed to convert seafood by- products into more profitable, marketable products. Many of these protein-rich seafood byproducts have wide range of dy- namic properties and potentially used in foods as binders, emulsifiers, and gelling agents.
Fish and fishery products represent a very valuable
source of protein and essential micronutrients for balanced
nutrition and good health. Fish silage or liquefied fish protein
is a simple way to convert fish bycatch and fish processing by-
products into very nutritious feedstuff. Value of waste fish can
be improved by converting it into fish protein hydrolysate
(FPH) by utilizing proteolytic enzymes to hydrolyze the fish
protein (Kristinsson and Rascso, 2000; Venugopal, 2006). The production of fish protein hydrolysate using endogenous en- zyme performed traditionally, such hydrolysates are pro- duced using the endogenous proteolytic enzymes present in muscles or fish viscera (Kristinsson and Resco, 2000). The en- dogenous enzymes trigger the breaking down of biomolecules to smaller peptides through autolysis process. Such process may either run at neutral or slightly alkaline pH, exploiting the serine protease of intestine in alkaline or the carboxyl pro- tease of gastric juice in acidic pH (Pastoriza et al., 2004). De- spite its availability in large quantities and it’s low cost, fish waste used as an excellent source of nutrients. When acidified, the viscera was stored and autolyze to yield a liquid of soluble peptide and essential amino acids (Ashraf, 2012).
The optimization process by conservative method is
monotonous, incomplete and requires a lot of time to complete a single experiment. The Response Surfaces Methodology (RSM) seems to be a very popular and effective method in the optimization and food process monitoring (Wangtueai and Noomhorm, 2009). It is the modern, statistically derived exper- imental design. This relates product treatment to the outputs and establishes a regression equation to describe inter- relations between input parameters and product properties and very cost effective (Cho et al., 2004).
Protein hydrolysates have a wide range of applica- tions in a various industries, including, human nutrition, ani-
mal nutrition, pharmaceuticals, cosmetics and fertilizers. Pro-
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1864
ISSN 2229-5518
tein hydrolysate generated from fish proteins as bioactive compounds is good source for nutritional supplements as it can be easily absorbed and utilized for diverse metabolic activ- ities (Nesse et al., 2011). It is also useful as a nitrogen source in the growth media for microorganisms (Duarte de Holanda & Netto, 2006; Quitain et al., 2001; Kristinsson & Rasco, 2000). Due to the high protein content, protein hydrolysates, extract- ed from marine by-products, have become well-accepted in the food industry (Cordova-Murueta et al., 2007).
However, until now no information reported regard-
ing the preparation and characterization of protein hydroly-
sate in Pellona ditchela. Hence, the objective of this study is de-
signed to investigate the optimum condition of protein hy-
drolysis from underutilized fish Pellona ditchela, to achieve
maximum degree of hydrolysis in acidic condition ( sulfuric: formic acid) through RSM design. Furthermore, the chemical composition and amino acid content of the protein hydroly- sate obtained in optimized hydrolysis condition can be ana- lyzed to identify the nutritional value of the product. The final fish protein hydrolysate from Pellona ditchela will serve as val- ue added product and will improve the economical value of this discarded fish. Hence the autolysis process in optimized condition obtained through RSM design will improve a nutri- tionally rich product and the bio-product obtained can serve
potential way to enhance the economic value of underutilized
(0, 73, 80, 90, 100, 106 mins). After each incubation period, the auto-hydrolysates are exposed to 800C for 15min to inactivate the endogenous enzymes. The heated hydrolysates centri- fuged at 6000rpm for 15min to obtain the corresponding su- pernatants (Ashraf, 2012). The supernatant was subjected to freeze-drying and the product obtained termed as fish protein hydrolysate was stored at -200C until further analysis.
2.4 Experimental of Response Surface Methodology
(RSM)
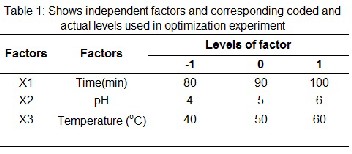
The hydrolysis conditions for Pellona ditchela were op- timized using response surface methodology (RSM) with a Central composite design. Three different independent varia- bles includes reaction temperature (X1, °C), reaction time (X2, minute), and pH (X3) were employed and the factor levels were coded as −1 (low), 0 (central point) and +1 (high) the dif- ferent factors and corresponding levels are presented in Table
1. The range for each independent variable was predetermined
based on results of preliminary study (data not shown). De-
gree of hydrolysis (DH) selected as dependent variables. The
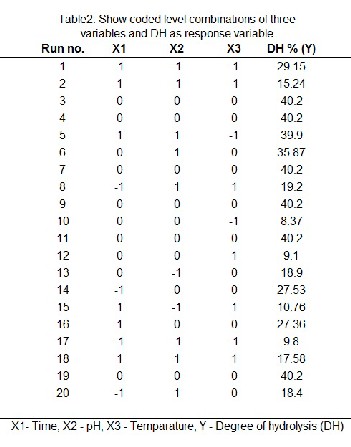
central composite design composed of 20 treatments which includes three factorial points, six axial points (α=1.68) and six replicates of the central point. The design of experiments and dependent variable values was presented in Table 1
fish.
IJSER
2. MATERIALS AND METHODOLOGY
2.1 Sample Collection
Pellona ditchela obtained from locality near to the coastal area of Marina for low commercial value. The fish samples packed in the sterile polyethylene bag, kept in ice and transported to laboratory.
2.2 Physical Analysis and Sample preparation
The samples were collected in ice-cold condition and subjected to physical analysis including the measurement of weight, length of the species, the texture of the skin; color and opacity of the corneal condition and gills were checked to understand the freshness of the sample. After analysis of physical condi- tion to evaluate the freshness, fish samples were rinsed and filleted down. The fillets were packed in polyethylene bags and freeze-stored at -20°C until used. The homogenate was obtained by mincing filleted tissues with water (in the ratio of
1:3) using high-speed blender for 2mins and preserved at -200c until further analysis.
2.3 Preparation of hydrolysate
The frozen homogenate was thawed and divided into 15 portions corresponding to time, pH and temperature respec- tively for the preparation of hydrolysate to undertake optimi- zation study. The acidic condition maintained in the presence of sulfuric acid: formic acid. The pH values ranging from
(3-6.6) were adjusted with 1N H2 S04 and 1N NaOH and the autolysis was carried out by maintaining at temperature of
(330C, 400C, 500C, 600C & 660C,) in orbital shaking at 125rpm, samples were withdrawn at different time interval for analysis
Randomized experimental runs were carried out with the purpose of minimizing the effect of unexpected variability in the observed responses (Wangtueai and Noomhorm, 2009).
2.4.1. Statistical Analysis
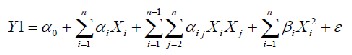
The response surface methodology (RSM) was statis- tically analyzed by Design-Expert, Version 8.0.7.1 software (Stat-ease Inc., Minneapolis, Minn., U.S.A.). The multiple re- gressions analysis was performed, as three parameters were varied, 10β-coefficients had to be estimated which included coefficients for the three main effects, three quadratic effects, three interactions and one constant (See et al., 2011). It was assumed that the estimated behavioral model of dependent variables was described by a second order polynomial equa- tion. Statistical analysis of the model was performed to evalu- ate the analysis of variance (ANOVA).
Where, α0 is the constant, αi is the linear effect of the input factor Xi, αij is the linear effect by linear interaction effect be- tween the input factors Xi and Xj, βi is the quadratic effect of
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1865
ISSN 2229-5518
input factor Xi and ε is the error (Benyounis et al., 2005). The optimization experiments for autolysis assisted hydrolysis and the responsiveness for the corresponding independent factors are exhibit is Table 2.
3 DEGREE OF HYDROLYSIS
As demonstrated in Degree of hydrolysis (DH) is defined as the proportion of cleaved peptide bonds in a protein hydroly- sate. It was estimated according to the TNBS method (Alder
4. ANALYSIS OF AMINO ACID PROFILE USING HPLC
Hydrolyzed samples with more DH value were deri- vatised prior to HPLC analysis. The total amino acids were analyzed by HPLC using C18 column at the flow rate of 0.5 ml/min with 338 nm Wavelength of detection (VWD) and reaction temperature 40°C.
5. RESULTS AND DISCUSSIONS
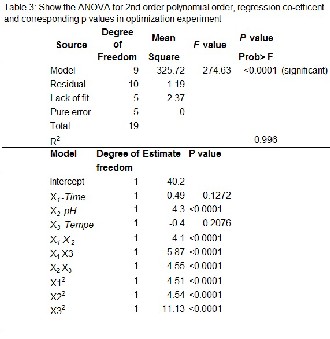
A central composite design was employed to determine the interaction of three factors (time, temperature and pH) in order to establish optimum condition for maximizing the degree of hydrolysis (DH%). The effect hydrolyzing time (X1 ), pH (X2 ) and temperature (X3 ) on the degree of hydrolysis (DH) (Y) was determined using Response Surface methodolo- gy (RSM). Based on Central Composite Design (CCD) 20 sets of hydrolysis experiments were conducted and the experi- mental data obtained is shown in Table 3.
IJSER
-
Nissen, 1979) using L-Leucine as standard the extent of
hydrolysis in the fish hydrolysate was calculated.
3.1. Proximate composition
The proximal composition of control and hydrolysate sample with maximum DH was determined according to AOAC (2005) methods. The total crude protein content was analyzed using Kjeldahl method (AOAC, 2005). The moisture content was determined according to oven method (AOAC,
2005). Lipid content of samples was estimated by Soxhlet ex- traction. Ash content was determined by charring the pre- dried sample in crucible at 600°C until a white ash was formed (AOAC, 2005). Estimation of protein was done with modified Bradford’s Method and the standard curve was plotted using standard protein BSA, the unknown protein concentration is plotted in standard curve.
The responsiveness of Y was evaluated through quadratic model and the response surface regression equation deployed was
Y= +40.20+0.49 X1 +4.30X2 -0.40 X3 +4.10 X1 X2-5.87X1 X3 -4.55
X2 X3 -4.51 X1 2-4.54 X22-11.1 X32
Where, Y, X1 , X2 and X3 are DH, time (mins), pH and tem- perature (°C) respectively.
5.1. Analysis of Variance - Conditions of optimum responses
The competence of the model is justified through
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1866
ISSN 2229-5518
analysis of variance (ANOVA), as shown in Table 4. The statis- tical significance of the quadratic polynomial model equation was evaluated through analysis of factor (F test) and ANOVA. The F-value implies that the second order polynomial model is significant to the analysed data and the coefficient of variation (CV) is 4.21% which signifies the extent of accuracy of the ex- periment. In general, lower the value of CV, higher the relia- bility of the experiment, here a lower value of CV indicated a better precision and reliability of data (Box et al., 1978).
regression model and interactive effects illustrated in 3D re- sponse surface plots. From the model, optimum condition re- quired for maximum DH was identified as, time (X1 ) of
90mins, pH(X2 ) 5 and temperature (X3 ) 500C. Models pre- sented showing figure 1 the effect of two factors, while the other factors were held in zilch effect. In the present study, DH value 40.1% was obtained from the model equation and the graph obtained displays in bell-shaped pattern in the response surface graph. The DH of the present analysis is in bell-shaped pattern, the comparable to outcome reported by Amiza et al.,
2012, the effects of pH and temperature on the DH, Sami Saidi
et al., 2013. From figure 1(a) it is clearly evident that the DH
increased with increase in pH and the effect of pH and tem- perature on DH is in bell-shaped pattern clearly visualize in figure (c).
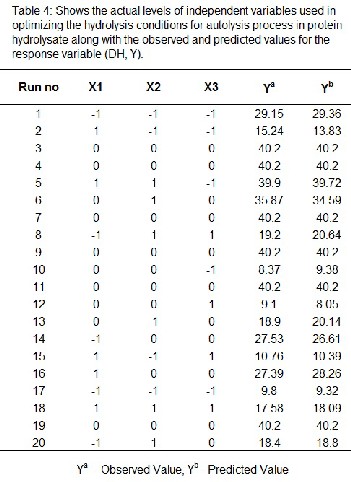
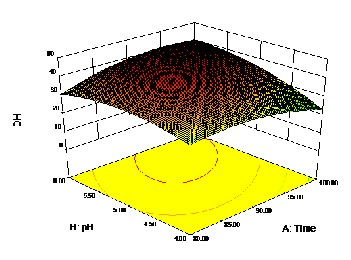
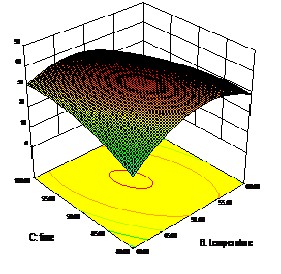
IJSEFigureR1: a) Effect of pH and Time on DH
The precision of a model was checked by the regres- sion coefficient (R2) and calculated as 0.9960, which indicated that the quadratic model can explain a high percentage of the variability i.e. 9960%. It can also suggest that, this quadratic model appropriately represent the real association among the chosen hydrolysis parameters. While fitting the model, vari- ous statistical analysis techniques were used to judge the ex- perimental error, the suitability of the model, and the statisti- cal significance of the terms in the model. The regression coef- ficients and corresponding P values are given in Table 4.
The mutual interaction between the optimum factors was implied through P values which act as tool to check the coefficient. For instance lower the P value, higher the signifi- cance of the corresponding coefficient. The interaction be- tween the independent factors has been described through
Figure 1: b) Effect of Time and Temperature on DH
The results showed a certain correlation between the mass distribution of soluble peptides and the DH. The results obtained showed these hydrolysis reactions with different conditions provided in general a high proportion of low mo-
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1867
ISSN 2229-5518
lecular weight peptides and free amino acids potentially up- gradable in food supplements.
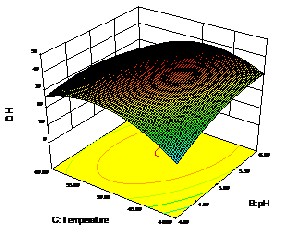
Figure 1: c) Effect of Temperature and pH on DH
5.2. Proximate analysis
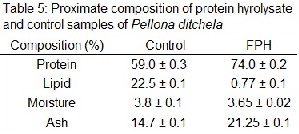
The proximate composition on fish protein hydroly- sate obtained in optimized condition and unhydrolysed sam- ples of P. ditchela mentioned in Table 5.
The hydrolysates obtained from, P.ditchela exhibits considerably high level of total amino acids. In the present experiment data, the hydrolysate endure rich in essential ami- no acid such as theronine, methionine, isoluecine and Phenyl alanine this indicate the hydrolysate is nutritionally rich prod-
ucts (figure 2). From the quantitative point of view, the ratio of
essential amino acids to non-essential amino acids was in- creased in P.ditchela tissue after hydrolysis process. Indeed protein hydrolysates developed by acid autolysis was rich essential amino acid, which includes arginine, tyrosine, and but lower in aspartic acid, serine and histidine. This finding was close to the report of Klompong et al., (2009), which showed that yellow stripe trevally (Selaroides leptolepis) pro- tein hydrolysates had a higher ratio of essential amino acids to non-essential amino acids. Shahidi and others (1995) reported that the composition of protein hydrolysates depended on the
Hydrolysate sample in optimum condition contains high protein content 74.0±0.2% whereas, in unhydrolsed sam- ple the percent of protein was comparatively low i.e. 59.0 ± 0.3
%. In contrast to protein, the lipid content of the prepared hydrolysate exhibits a converse range when compared to un- hydrolysed control sample mainly due to the interference of acid in hydrolysis. In acidic hydrolysis, free lipids are cross- linked to proteins and then break down protein particles, so that most lipids are accessible to solvent. The Lipid residues in product must be lower than 0.5% to prevent alteration of the lipid fraction during storage (Spinelli et al., 1972) the reduction in lipid content in protein hydrolysate is due to the exclusion in centrifugation and mostly due to lipid oxidation. This can improve the product stability and quality (Shahidi et al., 1995; Diniz and Martin 1997; Kristinsson and Rasco 2000b: Ali et al.,
2010).
5.3. Amino acid composition
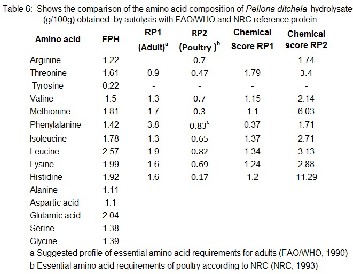
The amino acid composition of fish protein hydroly- sate and compute chemical score of samples of P.dictchella are presented in the Table 6.
type of enzyme used.
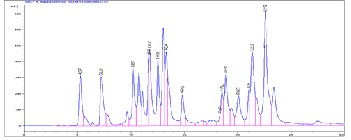
Figure 2: Chromatogram of protein hydrolysate obtained from
Pellona ditchela
Shahidi et al., 1995 also reported autolytic method can be suggested for recovery of proteins either from underuti- lized or fish processing waste. Moreover, due to simplicity of the operation and no enzyme costs involved the process still used for the preparation of protein hydrolysate. The protein level in the hydrolysate is influenced by the hydrolysis time, pH and temperature, in the present paper, the optimum con- dition used for tissue autolysis was determined as 90mins,
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1868
ISSN 2229-5518
pH5 and temperature 500Cand is evident from the response surface Figure 1.
The amino acid composition in this study revealed that the amino acid profiles of the P.dictehla hydrolysates generally higher in EAA compared to the suggested pattern of require- ment by FAO/WHO for the adult humans. The results of the P.dictehla chemical score showed that the composition in EAA of fish hydrolysates exhibits higher than the amount required the FAO/WHO and NRC standards, the hydrolysates fulfill human requirements and poultry feed. Based on the results in spite of minor deficiencies in certain essential amino acids the protein hydrolysate does no lose its nutritional value, conse- quently it can be considered as a balanced ingredient in food. This is an interesting result, since the nutritional composition, especially the essential amino acid content is a determining factor in human and poultry feeding. The outcome of this study indicates that the protein hydrolysate from P.dictehla may potentially serve as a good source of desirable peptides and amino acids.
Nutritive value of a protein can be estimated by its chemical score which aids in comparing the levels of essential amino acids between the test and the standard. In the present experiment chemical scores are computed based on the refer- ence of FAO/WHO (1990) for adults. In fact, proteolysis led to
the liberation of additional amino acids compared to raw ma-
7. REFERENCES
[1] A. Zynudheen, G. Ninan, A. Sen, and R. Badonia. “Utilization of trawl bycatch in Gujarat (India)”. NAGA, World Fish Center Quarter- ly, 27(3), 20-232004.
[2] Ali Motamedzadegan1, Bahareh Davarniam, Gholamhassan Asadi,
Abdolmohammad Abedian and Mahmoudreza Ovissipour. “Opti- mization of enzymatic hydrolysis of yellow fin tuna Thunnus albacares viscera using Neutrase”. Int Aquat Res, 2: 173-181, 2010.
[3] AOAC. Official Methods of Analysis. 16 ed. Association of Official
Analytical Chemists Washington, Washington DC. 2005.
[4] Ashraf A. Khalil. “Protein Characterization of the Aqueous Soluble Phase of Acidified and Autolyzed Bolti Fish (Tilapia nilotica) Vis- cera”. Asian Journal of Biotechnology, 4: 108-119. 2012..
[5] F. Shahidi, X. Q. Han, and J. Synowiecki. “Production and character-
istics of protein hydrolysates from capelin (Mallotus villosus)”. Food
Chemistry, 53, 285–293, 1995.
[6] FAO. “Fact Sheet”: The international fish trade and world fisheries' FAO, Rome. 2010.
[7] FAO/WHO. Energy and protein requirements. Report of joint FAO/ WHO/UNU. Expert Consultation Technical Report. 1990.
[8] G.E.P Box, W.G. Hunter, J.S. Hunter. Statistics for experimenters.
Wiley, New York. 1978.
[9] H. Duarte de Holanda, and F. M. Netto. “Recovery of components from shrimp (Xiphopenaeus kroyeri) processing waste by enzymatic hydrolysis”. Journal of Food Science, 71(5), 298–303 2006.
IJSER
terial and to the destruction or complexation of some amino acids. It is quite acceptable that conducting the hydrolytic re- actions at ambient temperatures, without adding exogenous enzymes can be effective and financially interesting alternative for protein recovery and dry matter solubilisation
5.4. Conclusion
Raw materials or wastes, if not utilized may cause environmental, health and economical problems. Hence, op- timal utilization of the raw material is of prime importance to cope for the increasing demand products. Influence of external parameters such as, time, pH and temperature on protein re- covery, observed during hydrolysis reaction. From RSM mod- el, the optimum condition required for maximum degree of hydrolysis (40.01%) was identified as, time (X1 ) of 90 mins, pH5 (X2 ) and temperature (X3 ) 500 C. The hydrolysate pre- pared in optimized condition with maximum DH, exhibits amino acid profile with high content of EAA for human re- quirement. Thus, hydrolysate obtained from the low value fish P.dictehla can serve as a nutritional source for various industri- al applications such as, feed and food purpose. Indeed the outcome of such research can upgrade the value of the dis- carded fish and provide incentive for commercial develop- ments leading to cost-effective production of potential by- product in large-scale.
6. ACKNOWLEDGMENT
The authors would like to express their sincere gratitude to Department of science and technology (DST) New Delhi, India for the financial support under grant.
[10] H. G. Kristinsson and B. A. Rasco. “Fish Protein hydrolysates: Pro-
duction, Biochemical and functional properties”. Critical Review in
Food Science and Nutrition, 40, 43 – 81.2000.
[11] H.G. Kristinsson, and B.A. Rasco. “Biochemical and functional properties of Atlantic Salmon (Salmo salar) muscle hydrolyzed with various alkaline protease”. Journal of Agriculture and Food Chemistry,
24:177- 187, 2000.
[12] J. Adler-Nissen. “Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzene sulfonic acid”. Journal of Ag- ricultural and Food Chemistry, 27, 1256–126, (1986).
[13] J. H. Cordova-Murueta, M. A. Navarrete-del-Toro, and F. L Garcia- Carreno. “Concentrates of fish protein by catch species produced by various drying processes”. Food Chemistry, 100, 05–711, 2007.
[14] J. Spinelli, B. Koury, and R. Miller. “Approaches to the utilization of fish for the preparation of protein isolates; enzymatic modification of myofibrillar fish proteins”. Journal of Food Science, 37, 604-608, 1972.
[15] K. O. Nesse, A. P. Nagalakshmi, P. Marimuthu, and Mamta Singh.. “Efficacy of a fish protein hydrolysate in malnourished children”, In- dian J. of Clinical Biochemistry, DOI 10.1007/s12291-011-0145-z, 2011.
[16] K.U. Siddiqui, M.A. Islam, S.M. H. Kabir, A.T. A., Ahmad, A.K.A.
Rahman, E.U. Haque, Z.U. Ahmed, Z.N.T. Begum, M.A. Hasan, M. Khondker, and M.M. Rahman. Encyclopedia of Flora and Fauna of Bangladesh, Freshwater Fish, 22, 17, 2007.
[17] K.Y. Benyounis, A.G. Olabi, M.S.J. Hashmi. “Effect of laser welding parameters on the heat input and weld-bead profile”. J. Mater. Proc. Technol. 165, 978-985, 2005.
[18] L.G. Pastoriza, M.L. Samperdro, J.J.R. Cabo, Herrera and M. Ber- nardez,. “Solubilisation of proteins from rayfish residues by endog- enous and commercial enzymes”, J.Sci. Food.Agri, 84: 83-88, 2004.
[19] M. A. Amiza, Y.L Kong, and A. L. Faazaz. “Effects of degree of hydrolysis on physicochemical properties of Cobia (Rachycentron canadum) frame hydrolysate”. International Food Research Journal,
19(1), 199-206, 2012.
[20] R. Pawar Prabhakar. “Assessment of Bycatch and Discards in Marine Capture Fisheries from Uran (Raigad), Navi Mumbai, Maharashtra”. The Ecoscan, 5(3&4), 105-109, 2011.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1869
ISSN 2229-5518
[21] R.L. Lewison, L.B. Crowder, A.J Read, and S.A. Freeman. “Under- standing impacts of fisheries bycatch on marine megafauna”. Trends in Ecology and Evolution, 11, 598-604, 2004.
[22] S. F. See, Hoo, L. L. and Babji, A. S.” Optimization of enzymatic hy- drolysis of Salmon (Salmo salar) skin by Alcalase”. International Food Research Journal. 18(4), 1359-136, 2011.
[23] S. M. Cho, K. S. Kwak, D. C. Park, Y. S. Gu, C. I. Ji, D. H. Jang, Y. B.
Lee, and S. B. Kim. “Processing optimization and functional proper- ties of gelatin from shark (Isurus oxyrinchus) cartilage”. Food Hydrocol- loids, 18: 573–579, 2004.
[24] S. Wangtueai, and A. Noomhorm. “Processing optimization and characterization of gelatin from lizardfish (Saurida spp.) scales”. LWT
- Food Science and Technology, 42,825–834, 2009.
[25] S. Y. K. Shenouda, and G. M. Pigott. “Lipid-protein interaction of fish protein: actin-lipid Interaction”, J. Food Sci, 40, 523-32, 1975.
[26] Sami Saidi, Marie-Pierre Belleville, Andre Deratani, and Raja Ben Amar.”Optimization of peptide production by enzymatic hydrolysis of tuna dark muscle by-product using commercial proteases”. Afri- can Journal of Biotechnology, Vol. 12(13), 1533-1547, 27, 2013.
[27] V. Klompong, S. Benjakul, D. Kantachote, and F. Shahidi. “Ant oxida- tive activity and functional properties of protein hydrolysate of yel- low stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type”. Food Chemistry, 102: 120-131, 2007.
IJSER
IJSER © 2013 http://www.ijser.org