ISSUE 2229-5518
Offshore Steel Structures Corrosion
Damage Model
Salau M. A, Esezobor D. E, Omotoso Matthew Folorunso
Department of Civil & Environmental Engineering, University of Lagos, Akoka, Yaba Lagos State, Nigeria
Department of Metallurgical & Materials Engineering University of Lagos, Akoka, Yaba Lagos State, Nigeria
Abstract: This paper reviews the current state-of-the-art modeling for marine steel structures chloride-induced corrosion. To investigate the effect of ocean wave on chloride accumulation in the neighborhood of offshore crude oil production platform. The model demonstrates that the period required for the steel structures built in marine environment to experience corrosion losses depends on chlorine ion diffusion rate, accumulation rate and time for corrosion to occur and generate enough rusting to fall off from the steel component surface. The work revealed that chlorine ion concentration in surrounding of offshore platform was higher than the open seawater due to crude oil production activities. The work provides estimation method for chloride accumulation rate in the neighbourhood of offshore production platform based on chlorine ion concentration, wave velocity and the age of platform.
Index Term: Chloride Accumulation, Chlorine-ion Concentration, Chloride Diffusion, Coating Life Span, Corrosion Damage, Crude
Oil Production, Offshore Platform Structures
—————————— ——————————
odeling durability of marine steel structures due to corrosion damage requires quantitative component understanding of the structure environment and steel
physical deterioration processes. Equations for each part of
these processes are available. However, several models that are available in the literature have been developed for a particular environment which is not suitable for offshore oil production platform associated with accidental ion discharge and continuous movement of ocean wave. This work has provided necessary modifications and extension to account for ocean wave and chloride accumulation in the neighbourhood of offshore platform due to crude oil production activities.
The process of chloride-induced corrosion for offshore steel
component is by diffusion of chlorides through the damaged coating while the chloride builds up with time on the steel component surface. Whenever the chloride attains critical threshold, the passive oxide layer on the steel breaks down and corrosion start [1]. The replacement of corroded component may be made, however the cycle continues on the new component.
The process of modeling requires the following details:
Calculating the chloride accumulation rate in the surroundings of offshore platform, determining the effect of ocean wave on chlorine ion concentration; establishing period at which steel components begin experiencing corrosion damage, as it is common to offshore jacket components.
Chlorine ion is one of the significant agents that responsible for corrosion process in marine environment. Coated steel develops passive oxide layer which is highly protective and grows at a slow rate. As long as the steel remains in good alkaline condition the passive layer will prevent corrosion initiation on the surface of the steel.
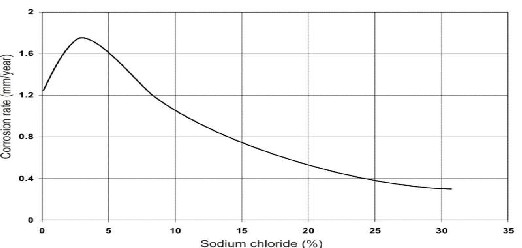
Seawater typically contains about 3.5% sodium chloride, although the salinity may be stronger in some areas. The rate of corrosion is controlled by the chloride content, oxygen, and seawater temperature. 3.5% salt content of seawater produces the most corrosive chloride salt solution [2]. Dry steel does not corrode even in the presence of chloride and below a relative humidity of 60% chloride- induced corrosion rate is negligible [3].
Crude oil production activities such as accidental discharge of drilling mould, waste water and other associated chloride substances into seawater attributes to chlorine accumulation around offshore platforms. The flaring gases consist of HCL gas among others and may form rain cloud above the platform and fall back around the platform as acidic rain. The above mentioned factors among others contribute to continuous chloride accumulation in the neighborhood of offshore oil production platforms.
Chloride ion is transport in solution through damaged steel
coating into the surface of steel members in several ways
which includes diffusion and water capillary process. Meijers (2003) developed a finite element analysis model that uses convection and conduction modeling of chloride transport process [1].
IJSER © 2011 http://www.ijser.org
ISSUE 2229-5518

Fig 1 Corrosion of Steel in Various Sodium Chloride Solutions
Pierre R. Roberge (1999)
However, most models assume that the dominant process is diffusion for a reasonably well-constructed structure with good quality of coating. Diffusion calculation is a reasonable approximation of the overall real process for chlorine ion transportation. The diffusion process is modeled by solving one dimensional equation for Fick’s second law of diffusion as given in Equation (1).![]()
![]()
(1)
where:
C = salt ion concentration
T = time
D = diffusion coefficient
Equation 2 can be derived from Fick's First law and the mass balance.![]()
![]()
![]()
![]()
(2)
If the diffusion coefficient D is constant, we can exchange
the orders of the differentiating and multiplying by the constant:![]()
![]()
![]()
![]()
(3)
In the case of diffusion in two or more, dimensions Fick’s
Second Law becomes![]()
(4)
In a condition in which concentration does not change by time, the above equation becomes zero which is Laplace's equation. This Equation is usually solved using the error function solution:![]()
(5)
where:
C(x, t) = salt concentration at depth x at time t
Co = surface concentration
erf = error function
= use for two or more dimensions
Fick’s second law of diffusion discussed earlier specified that chloride ion concentration (C0) should remain constant through the diffusion process. However, the surroundings of offshore production platforms are characterized with accidental discharge of ions which makes chlorine ion increases with time.
The value for the rate of chloride accumulation (m) is not readily available for marine structures [5]. However, this
paper demonstrates that this value can be estimated if the time the platform was built is known and the seawater chlorine ion concentration in the neighborhood of the platform is known by carrying out chemical analysis of the seawater.
Fick's second law predicts how diffusion causes the concentration to change with time. To account for the increasing in C0, it was assumed that C0 increases linearly and satisfying following Equations:
(6)
IJSER © 2011 http://www.ijser.org
ISSUE 2229-5518
Where m is the rate of chloride accumulation and t is the age of the offshore platform. The solution to Fick’s second law is modified as follows to account for the variation of C0 along the seawater depth.
when:
(11)
= 0, (12)
![]()
(7)
It was established in Equation (7) that chlorine ion concentration (C0) increases with the platform age (t) and the rate of chloride accumulation (m). However, this equation may further be modified as a result of ocean wave that constantly moving water away from neighborhood of
when:
> 0 (13)
offshore platform. Therefore, to account for the decreasing C0 as a result of ocean wave, it is assumed that C0 reduces directly proportional to the ocean wave velocity. The relationship between wave velocity, area of flow and volume of water can be represented mathematically in Equation (8).![]()
![]()
(8)
where is the seawater density and W – mass flow rate; For the purpose of straightforward derivation, the volume of flow water (Q) is assumed to be linear and the flow area (A) is 1m2 then, Equation (8) can re-write as:![]()
(9)
The solution to Fick’s second law is therefore modified to account for ocean wave velocity:![]()
(10)
Based on the field data collected during this study, the declining of C0 due to ocean wave was presumed to be decreasing linearly, satisfying Equation (11) include other conditions stipulated in Equations (12) and (13) respectively.
Where v is ocean wave velocity, m is the rate of chlorides accumulation and Cw is for open seawater chlorine ion concentration which is about 3.5% in Niger Delta region. Chloride accumulation rate on the seawater surface can be estimated using Equitation (14), if chlorine ion concentration of the seawater is known based on seawater sample analysis with the offshore platform age.
(14)
Table 1 shows wave data in Niger Delta region of Nigeria. These values were used in the calculation of chloride ion concentration based on the Metocean direction, platform age of 21 years with the assumption that chloride accumulation rate in the platform surrounding was
1.7g/Liter/year.
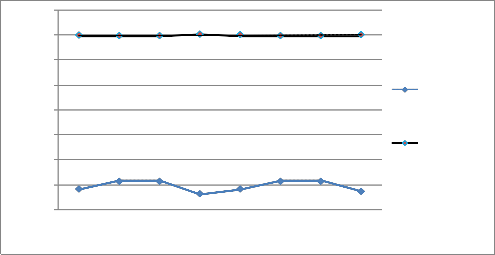
The results were presented in Fig 2, which revealed that the
estimation of chloride accumulation rate is higher in the
area with lower ocean wave velocity and lower in the area with higher ocean velocity. Table 2 also presented the rate of chloride accumulation in the surrounding of several platform investigated in the study based on average ocean wave velocity of 0.41m/s with the applicable ages of each platform using Equation (14).
S/N | Metocean Direction (0o) | Wave Period (s) | Wave Velocity (m/s) | Wave Length () (m) |
1 | 20 | 14 | 0.31 | 4.34 |
2 | 65 | 13 | 0.57 | 7.41 |
3 | 110 | 5 | 0.57 | 2.85 |
4 | 155 | 5 | 0.31 | 1.55 |
5 | 200 | 5 | 0.41 | 2.05 |
6 | 245 | 5 | 0.57 | 2.85 |
7 | 290 | 5 | 0.57 | 2.85 |
IJSER © 2011 http://www.ijser.org
ISSUE 2229-5518
4
4
3.5
3
2.5
(%) 2
1.5
1
0.5
0
20 65 110 155 200 245 290 335
Metocean Direction (degree)
Wave Velocity
(m/s)
Surface Chlorine-ion concentration (%)
S/N | Facilities Description | Facility Age (yr) | Wave Velocity (m/s) | Chlorine ion Concentration g/Liter | Chloride Accumulation g/Liter/yr |
1 | Offloading Buoy | 12 | 0.41 | 38.0 | 3.2 |
2 | Jacket Platform - A | 13 | 0.41 | 35.8 | 2.8 |
3 | Jacket Platform - B | 10.5 | 0.41 | 35.1 | 3.4 |
4 | Jacket Platform - C | 20 | 0.41 | 36.2 | 1.8 |
5 | Jacket Platform - D | 23 | 0.41 | 38.0 | 1.7 |
V m
Chloride Accumulation (m)
Wave
Velocity (V)
3.5% 3.8%
Chlorine ion concentration (CO)
IJSER © 2011 http://www.ijser.org
ISSUE 2229-5518
The period required for the steel structures in marine environment to experience corrosion damage is equal to the time needed for chlorine ion to diffuse down to the steel component surface, accumulate in concentration in excess of the corrosion threshold. Plus the time for corrosion to occur and generate enough rust material to fall off from the steel component surfaces.
Also the rate of marine steel component corrosion losses is
directly proportional to the amount of rust generated and falls off from the member surfaces.
One quantitative equation to determine the rate of production of rust is given by Liu (1996) as shown in Equation (15) [4].![]()
(15)
where: = molecular weight of steel or corrosion products icorr(t) = rate of corrosion as a function of time, t = time,
∆crit = critical volume of corrosion product required to fall
off.
The time required to generate the volume of rust is obtained by solving Equation (15). This model was validated in laboratory and the problem with using this model is that once corrosion initiates, the corrosion rate varies with time and the appropriate way is to measure corrosion rate several times using average of measured corrosion rates.
This method is frequently used in petroleum industry for
the assessment of offshore jacket platform structures. The period for steel component to experience corrosion losses can be represented mathematically as given in Equation (16) and (17) for uncoated and coated marine steel structures respectively.
Td Ti tp (16)
Td Ti tp (17)
where Td – time for corrosion losses, Ti – time for chlorine ion accumulation in excess of corrosion threshold tp – time for corrosion occurrence and rusting to fall off and - coating life span.
Chlorine ion has been identified in this work as one of the major agents that responsible for marine steel structures corrosion damage. The rate of steel component corrosion losses in an offshore environment is directly proportional to the amount of chlorine ion deposited on the steel component surface. The above description is valid for coated and uncoated steel structures. However, the time to
corrosion damage for coated structures is longer by coating life span (). This assumption is valid provided the coating is intact and does not allow chlorine ions diffusion to the steel surface.
Seawater contains chloride in certain proportion and the
amount is higher in the neighborhood of offshore platforms due to crude oil production activities. This paper revealed that the rate of chlorine ion concentration around offshore platforms is directly proportional to the rate of chloride accumulation and indirectly proportional to ocean wave velocity and this value will not less than open seawater chlorine ion concentration of 3.5% (35g/L) in Niger Delta. The chlorine ion concentration value was higher around offloading Buoy and jacket platforms based on the seawater analysis. These values with each facility age and average wave velocity of 0.41m/s employed to calculate the chloride accumulation rate around the platforms as presented in Table 2 using Equation (14).
Fig 2 presented ocean wave velocity regarding Metocean
directions. The chlorine ion concentration was found to be higher in the area of lower ocean wave velocity and lower in the region of higher ocean wave velocity. Therefore, the rate of corrosion may vary on different steel component located at different Metocean direction that belongs to the same platform structures.
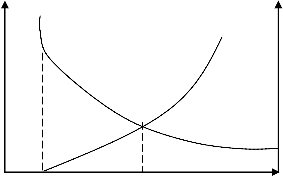
Fig 3 demonstrates that the rate of chlorine ion
concentration increases from initial value of 3.5% as chloride accumulation rate increases. Also, the Fig showed chlorine ion concentration reduction as the ocean wave velocity increases. However, this value was not less than the open seawater chlorine ions concentration of 3.5%. The equilibrium value of 3.8% (38g/L) at inter-section between wave velocity and chlorides accumulation graphs was the constant chlorine ion concentration around offloading buoy provided the rate of chloride accumulation and wave velocity is unchanged.
Fick’s second law of diffusion has been extended to account for continuous accumulation of chloride in the neighbourhood of production platform in an offshore environment. The ocean wave effect on chlorine ion concentration in the vicinity of offshore platform was also presented. The work has provided accurate index representation of chlorine ion distribution based on Metocean directions. This phenomenon has given hint to various degree of component corrosion damaged due non uniformity of chlorine ion concentration around offshore structures. The period required for the marine steel structures to experienced chloride induced-corrosion damage was established. This accomplishment is significant
IJSER © 2011 http://www.ijser.org
ISSUE 2229-5518
for appropriate inspection and assessment of offshore platform structures with regards to safety, which guarantee
[1] Meijers, S. J. H., Computational Modeling of Chloride
Ingress in Concrete, 2003, Delft University.
[2] Pierre R, Roberge Distance Learning Corrosion Course, CCE281, Impact Principle and Practical Solution 1999.
[3] Bentz, E. C., ―Probabilistic Modeling of Service Life for
Structures Subjected to Chlorides,‖ ACI Materials Journal.
2003.
continuous crude oil production in an off6shore environment.
[4] Manual on Service Life of Corrosion-Damaged Reinforced Concrete Bridge Superstructure Elements Transportation Research Board 2006 Executive Committee [5] Liu, Y., Modeling the Time-to-Corrosion Cracking of the Cover Concrete in Chloride Contaminated Reinforced Concrete Structures. 1996, Virginia Polytechnic Institute and State University
[6] Eastern Nigeria Shallow Water Metocean Criteria:
Version 1.2, April 2002.
IJSER © 2011 http://www.ijser.org