The research paper published by IJSER journal is about Molecular adducts of pentafluorophenylantimony (III) chlorides (Rf) n SbCl3-n with neutral monodentate oxygen, nitrogen and sulphur donors 1
ISSN 2229-5518
Molecular adducts of pentafluorophenylantimony (III) chlorides (Rf) n SbCl3-n with neutral monodentate oxygen, nitrogen and sulphur donors Isha Rizvi, Amarika Singh, Navneet Singh, Dharmendra K. Srivastava
Abstract—Penta-coordinated and Tetra-coordinated neutral adducts (C6F5)2SbCl.L, (C6F5)SbCl2.L and (C6F5)SbCl2.L2 [L= Pyridine, Thiourea, Triphenylarsenic oxide, Hexamethyl phosphoramide, Triphenylphosphine oxide, α-Picoline, β-Picoline, γ-Picoline] have been synthesized .Molecular adducts are monomeric in benzene and non-electrolyte in acetonitrile. IR spectra and conductance measurement suggest that the complexes are neutral..
Keywords— Adducts, bis (pentafluorophenyl) antimony (III) chloride, characterised, Lewis acidity, monomeric, non-electrolyte, pentafluorophenyl antimony (III) dichloride.
—————————— ——————————
1 INTRODUCTION
Acceptor properties of antimony (III) halide and organo substituted antimony(III) halide have not been studied to considerable extent. Antimony (III) due to presence of three halo group behaves as strong Lewis acid and report on the
synthesis of neutral adducts and anionic complexes with enhanced coordination no. up to 4 to 5 are usually encountered and have been reported [1-7]. These reports have mainly been of academic interest and have not been exploited for other organo group substituted antimony (III) halide. Successive replacement of chloride group by phenyl and methyl group still leaves for example; RnSbCl3-n (n=1&2) with considerable Lewis acidity. Phenyl substituted antimony halide are better Lewis acid compared to methyl analogous. However, replacement of methyl group by CF3 enhances the Lewis acidity of complexes with different Ligands have been prepared [6, 7]. The acceptor properties of PhSbCl2 and Ph2SbCl have been investigated by Nottingham & workers toward oxygen, nitrogen and sulphur donars.PhSbCl2 forms complexes both 1:1 and 1:2 stoichiometry while Ph2SbCl form only 1:1 complexes [6].
The neutral, anionic and cationic complexes of Ar2SbCl and
PhSbCl2 have been prepared by us but the coordination number of the central metal atom does not exceed beyond five.
As has been mentioned above replacement of methyl group from MeSbCl2 by trifluoromethyl group enhances the Lewis acidity, it was considered worth while to examine whether replacement of an aryl group (C6H5, p-CH3C6H4) by pentafluorophenyl group (Rf =C6F5) will enhance the Lewis acidity to such an extent so as to facilitate the formation of complexes in which coordination number of central metal up to five.
————————————————
 Author name is Isha Rizvi currently pursuing Ph. D .in Chemistry from Institute of Engineering & Technology, Constituent college of GBTU, Sitapur Road, Lucknow, U.P., India, PH-8957362201. E- mail:isha.rizvi007@gmail.com
Author name is Isha Rizvi currently pursuing Ph. D .in Chemistry from Institute of Engineering & Technology, Constituent college of GBTU, Sitapur Road, Lucknow, U.P., India, PH-8957362201. E- mail:isha.rizvi007@gmail.com
The present work deals with the preparation and isolation of neutral adducts of (Rf)2SbCl and (Rf)SbCl2 (Rf=C6F5) with oxygen, nitrogen and sulphur donors .Two series of compounds were obtained in 1:1 and 1:2 stoichiometry . However, (Rf)2SbCl does not form complexes with 2 moles of ligand .The complexes have been characterised by elemental analysis, molar conductance and molecular weight data. The structure of the complexes has been assigned on the basis of IR and NMR spectra.
2 RESULTS AND DISCUSSION
Under anhydrous oxygen free condition neutral adducts of type ( I ,III ,V, VII , IX , XI,XIII , XVI ), could be obtained by interaction of bis(pentafluorophenyl) antimony(III) chloride , (C6F5)2SbCl and pentafluorophenyl antimony (III) dichloride with an equivalent of neutral monodentate ligand in ethanol and chloroform (Eq.1).
EtOH + CHCl3

(C6F5)2SbCl + L (C6F5)2SbCl.L (1)
Where L: Pyridine (Py), Picoline (α-C6H7N ,β-C6H7N & γ -
C6H7N), Triphenylarsenic oxide (Ph3AsO) , Thiourea (TU) , Triphenyl phosphine oxide (Ph3PO) , Hexamethyl phosphoramide (HMPA).
The adducts of type (II ,IV , VI , VIII , X , XII , XIV , XV , XVII ,
XVIII ) could be obtained by interaction of pentafluorophenyl antimony(ш) dichloride with 1:2 ( for II , XV , XVII ) or in appropriate stoichiometry of neutral monodentate liquid in ethanol and chloroform (Eq. 2 and 3).
for Compound No. II, XII, XVIII
EtOH + CHCl3

(C6F5)SbCl2 + 2L (C6F5)SbCl2.2L (2)
for Compound No. IV, VI, VIII, IX, XII, XIV, XVII
EtOH + CHCl3

(C6F5)SbCl2 + L (C6F5)SbCl2.L (3)
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Molecular adducts of pentafluorophenylantimony (III) chlorides (Rf) n SbCl3-n with neutral monodentate oxygen, nitrogen and sulphur donors 2
ISSN 2229-5518
Where L : Pyridine (Py) ,α- Picoline , β- Picoline , γ- Picoline(Pic) ,Triphenyl phosphine oxide (Ph3PO) ,Hexamethyl phosphoramide (HMPA) , Thiourea (TU).
All the reactions were found to proceed smoothly under mild condition. The products can be crystallized from petroleum ether (40-60o) or diethyl ether. In general mp of the adducts or complexes are high and few of them were found to decompose without melting. These adducts are readily soluble in polar solvents. These are stable toward atmospheric oxygen and moisture.
These adducts are monomeric in freezing benzene. Conductance measurement value of 10-3M solution of adducts in acetonitrile suggest the absence of ionic species.
2.1 IR Spectroscopy
The IR absorption due to C6F5 group attached to antimony are almost identical and do not differ significantly from those observed for pentafluorophenyl (III) compound earlier [8, 9,
10].
2.1.1 IR spectra of the adducts with oxygen donors
The IR absorption characteristic of C6F5 group ν(Sb-C) and (Sb- Cl) stretching frequency prepared in present paper resemble well with earlier values [8] and are listed in Table 4.
In case of oxygen donor ,Ph3PO, ν(P-O) appearing at 1192cm-1
in the free ligand show a negative shift and appear around
1130 +5 indicating co-ordination through oxygen atom of ligand [11].
In case of Ph3AsO complexes , the characteristic ν(As=0)
[12,13], lying at 880 cm-1 in the spectra of the free ligand undergoes a distinct negative shift on complexation .The corresponding absorption in the spectra of the adduct appears at 835+5cm-1 suggesting coordination from oxygen atom of the base.
2.1.3 IR spectra of adducts with sulphur donor
In case of thiourea complexes, ν C=N and ν C=S do not suffer any significant shift compared to free ligand. The positive shift of ν (N-H) from 3300 cm-1 in free thiourea to 3375cm-1 in the adducts indicates coordination through sulphur atom of the ligand[11].
As expected, bands associated with antimony-chlorine modes moves to lower energy on complexation e.g. from 340-320 cm-
1 in (C6F5)SbCl2 to 315+5 cm-1 is shifted to290+ 10 cm-1 [2, 6].
2.2 1H NMR, 13C NMR & 19F NMR spectra
2.2.1 1H NMR Spectroscopy
The 1H NMR spectra of compound no I, II shows similar multiplet for pyridine in range of δ 7.36 ppm to 8.98 ppm in which proton at 4th position shows most downfield peak, are characteristic for aromatic protons attached by pyridine in pyridine complexes. Compound no III, V, VII, IV, VI,VIII exhibits most shielded peak for protons of methyl group ranges from δ 2.31 to 2.53 ppm and a multiplet ranges from δ
7.18 ppm to 8.69 ppm are characteristic for picoline complexes. Compound no IX, X exhibits multiplet for phenyl group attached with arsenic atom in range of δ 7.30 ppm to 7.45 ppm. Compound no XI, XII also shows multiplet for phenyl group attached with phosphorus atom in range of δ 7.45 to 7.47 ppm. Compound no XIII, XIV; XV exhibits two signals at δ 2.42 ppm and δ 2.32 ppm due to –N-CH3 protons which is characteristic for HMPA complexes [14]. Compound no XVI, XVII, and XVIII exhibit singlet at δ 9.53 ppm shows that protons at nitrogen atom are some what similar to aldehydic protons which are deshielded and gives downfield peak characteristic for thiourea complex.
All the 1H NMR spectra of aforesaid compounds are taken in
CDCl3 as solvent and tetramethylsilane as reference material.
13
In case of HMPA complexes , the characteristic ν(p=0)
2.2.2
C NMR Spectroscopy
vibration appearing at 1218cm-1 in free ligand is considerably lowered to 1140+ 2 on complexation indicating coordination through oxygen of base [11].
2.1.2 IR spectra of adducts with nitrogen donor
Infrared absorption for all the isolated compounds associated with the antimony-pentafluorophenyl group and the aromatic donors overlaps and presents a complex picture which makes proper assignment considerably difficult .However bands due to aromatic donors which are characteristic have been identified to a certain extent.
Thus, pyridine out of plane deformation[6]observed at 404 cm-
1 in the free ligand , is shifted to 414+5 in (C6F5)SbCl2 and (C6F5)2SbCl complex .Similarly ,in plane deformation mode which appear at 604+2 cm-1 in the 1:1 and 1:2 complexes . The characteristic ν C=N vibration in the adduct with nitrogen donors (pyridine and Picoline) reported to appear in the range
1568-1575 cm-1 in free ligand [11] undergoes a positive shift on complexation and appear at 1605+15 cm-1 suggesting co- ordination through nitrogen atom of the base.
The 13C NMR or CMR spectra of all the complexes exhibit
signal at δ 101 ppm for C1, 148 ppm for C2 C6, 137.3 ppm for C3
C5 and 143.3 ppm for C4 atom of pentafluorophenyl group
attached with antimony. Compound no I, II exhibit additional
peaks at δ 149.9 ppm for C2 C6, 124.5 ppm for C3 C5, and 137.8 ppm for C4 atom which are characteristic for pyridine complexes. Compound no III, V, VII, IV, VI, VIII exhibit peaks ranges from 18.0 ppm to 23.9 ppm for methyl carbon, 148.6 to
158.0 ppm for C2 C6, 120.9 to 133.9 ppm for C3 C5, 136.0 to 147.0
ppm for C4 atoms, are characteristic for picoline complexes. Compound no IX, X exhibits peaks at 129 ppm for C1 C2 C6,
128.7 ppm for C3 C4 C5 atoms of triphenylarsine oxide complexes for their phenyl group attached with arsenic atom. Compound no XI, XII exhibit peaks at 133.1 ppm for C1, 132.3 for C2 C6, 128.8 for C3 C5 and 134.2 ppm for C4 atoms are characteristic of triphenylphosphine oxide complexes. Compound no XIII, XIV, XV exhibits peaks at 36.5 ppm characteristics for HMPA complex and in last compound no XVI, XVII, XVIII exhibits peaks at 176.7 ppm characteristic for
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Molecular adducts of pentafluorophenylantimony (III) chlorides (Rf) n SbCl3-n with neutral monodentate oxygen, nitrogen and sulphur donors 3
ISSN 2229-5518
thiourea complexes.
All the 13C NMR spectra of aforesaid compounds are taken in
tetramethylsilane as reference material.
2.2.2 19F NMR Spectroscopy
The 19F NMR spectra showed signal at δ -122.40 ppm, -146.20 ppm and -152.06 ppm corresponding to F2, 6, F4 and F3, 5 respectively.
2.1 Stereochemistry of neutral molecular adducts
On the basis of elemental analysis, IR, NMR spectra, molecular weight and conductance measurement, the neutral molecular adducts can be assigned tetra coordinated and penta- coordinated structure as shown in figure 1, 2 & 3. Similar structures have earlier been assigned for 1:1 and 1:2 addition products with R2SbCl and RSbCl2 respectively [2], [6], [15].
3 Experimental
Bis(pentafluorophenyl)antimony(III) chloride, (C6F5)2SbCl and(pentafluorophenyl)antimony(III) dichloride (C6F5)SbCl2 was prepared by reported method [16] through redistribution reaction. All the reagents were of reagent grade and used without further purification. The solvents were purified and dried before use. All manipulations were conducted in an atmosphere of nitrogen and stringent precautions were taken
to exclude moisture.
60ºC).
3.3 Reaction of (C6F5)SbCl2 with Ph3Ph3O (XII)
A solution of (pentafluorophenyl)antimony(III) dichloride,
(C6F5)SbCl2 (0.360 g, 1mmol) in chloroform (30 cm3) and
triphenylphosphine oxide (0.278 g, 1mmol) in ethanol (30 cm3) were stirred together at room temperature for 4 h. It was then filtered and filtrate on concentration in vacuo yielded a light green solid characterized as (pentafluorophenyl)antimony(III) dichloride- triphenylphosphine oxide adduct was recrystalized from petroleum ether (40-60ºC).
3.4 Reaction of (C6F5)SbCl2 with HMPA (XV)
A solution of (pentafluorophenyl)antimony(III) dichloride,
(C6F5)SbCl2 (0.360 g, 1mmol) in chloroform (30 cm3) and
HMPA (0.358 g, 2mmol) in ethanol (30 cm3) were stirred together at room temperature for 6 h. It was then filtered and filtrate on concentration in vacuo yielded a solid which was recrystalized from petroleum ether (40-60ºC) to afford (pentafluorophenyl)antimony(III) dichloride-HMPA adduct.
4 Figures

Rf
Cl
Analytical data are given in Table 1 & 2.Conductivity data Table 3 were obtained in acetonitrile with the help of a Philips magic eye type PR 950 Conductivity Bridge using a dip type conductivity cell. Molecular weights were determined cryoscopically in benzene given in Table 3. IR spectra were recorded on a Perkin Elmer 577 spectrophotometer in the range 4000-200 cm-1 Table 4 & 5. Typical experimental details of the reactions are described below. All the complexes were prepared in similar fashion.
3.1 Reaction of (C6F5)2SbCl with Pyridine (I)
A solution of bis(pentafluorophenyl)antimony(III) chloride,
(C6F5)2SbCl (0.491 g, 1mmol) in chloroform (30 cm3) and pyridine (0.079 g, 1mmol) in ethanol (30 cm3) were stirred together at room temperature for 4 h and then refluxed for 1 h to ensure complete reaction. It was then filtered and the filtrate was distilled off to remove excess of solvent to get viscous solid of bis(pentafluorophenyl)antimony(III) chloride- Pyridine adducts. It was recrystalized from petroleum ether (40-60ºC) to afford bis(pentafluorophenyl)antimony(III) chloride- Pyridine adducts.
3.2 Reaction of (C6F5)2SbCl with Ph3AsO (IX)
To a solution of bis(pentafluorophenyl)antimony(III) chloride,
(C6F5)2SbCl (0.491 g, 1mmol) in chloroform (30 cm3), triphenylarsine oxide (0.322 g, 1mmol) in methanol (30 cm3), was added drop wise. The reactants were stirred for 4 h at room temperature under nitrogen and the refluxed further for
24 h. It was then filtered and filtrate on concentration in vicuo
yielded a off white solid characterised as bis(pentafluorophenyl)antimony(III) chloride- triphenylarsine oxide adduct was recrystalized from petroleum ether (40-
IJSER © 2012
http://www.ijser.org
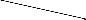 Cl
Cl

Sb





















L
Fig: 1 Rf SbCl2.L

Rf

Cl Cl
Sb
L
L
Fi g: 2 Rf SbCl2.L2
Rf
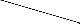
Rf  Cl
Cl

Sb





















L
Fig: 3 (Rf )2SbCl.L
The research paper published by IJSER journal is about Molecular adducts of pentafluorophenylantimony (III) chlorides (Rf) n SbCl3-n with neutral monodentate oxygen, nitrogen and sulphur donors 4
ISSN 2229-5518
5 Tables
Table1 Analytical data for bis(pentafluorophenyl)antimony(III)
chloride neutral adducts.
Table 3 Table 3 Molecular weight, conductance measurement of molecular adducts of bis(pentafluorophenyl) antimony(III) chloride and pentafluorophenylantimony(III) dichloride

Co mp oun d.

Adduct/Comp lexes
Mp
(°C)
Yield
(%)

Analysis: Found(Calculated:%)
C H N
Compound No. Molar conductance in
acetonitrile
(ohm-1cm2mol-1)
Molecular weight in nitrobenzene found(Calculated)
No.
I (C6F5)2SbCl.Py 178 59 35.75(35.
79)
0.86(0.
88)
2.45(2.
46)
I 4.6 568.2(570.43)



II 5.8 515.65(517.92) III 3.2 583.2(584.45) IV 6 450.65(452.85)
III (C6F5)2SbCl.α-
Pic

V (C6F5)2SbCl.β- Pic
IX (C6F5)2SbCl.
Ph3AsO

XIII (C6F5)2SbCl.H MPA
179 68 36.90(36.
99)
170 57 36.90(36.
99)
240d 72 44.25(44.
29)
105 67 32.23(32.
24)
1.20(1.
21)
1.20(1.
21)
1.85(1.
86)
2.70(2.
71)
2.38(2.
40)
2.48(2.
40)
----
6.25(6.
27)






V 5.6 582.2(584.45) VI 5.8 449.65(452.85) VII 4.8 581.2(584.45) VIII 5.9 450.65(452.85) IX 2.3 812.12(813.56) X 2.4 679.57(681.95) XI 3.6 767.2(769.61) XII 3.6 635.65(638.01) XIII 4.4 668.2(670.53) XIV 4.4 536.65(538.92) XV 3.86 716.65(648.86) XVI 5.4 568.2(567.45)
XVI (C6F5)2SbCl.TU 230d 74 27.50(27.
52)
0.70(0.
71)
4.92(4.
94)
XVII 5.6 434.65(435.84)

XVIII 4.6 509.65(511.96)
Table 2 Analytical data for (pentafluorophenyl)antimony(III)
dichloride neutral adducts.
Table 4 Table 4 IR spectra for (Rf)nSbCl3-n.mL (cm-1) (n = 2; m =
1, 2

Compo und. No.
Adduct/Com plexes
mp(° C)
Yield(
%)
Analysis: Found

(Calculated:%)
C H N

Compound
No.
ν(Sb- C)
ν(Sb- I)/ ν(Sb-
ν(C=N)/ ν(P=O)/ (N-H)/(As-O) ligand (complex)
II (C6F5)SbCl2.2

Py
IV (C6F5)SbCl2.α
Pic

VI (C6F5)SbCl2.β- Pic
VIII (C6F5)SbCl2.γ- Pic
X (C6F5)SbCl2.

Ph3AsO
XII (C6F5)SbCl2.P
h3PO

XIV (C6F5)SbCl2.H MPA
XV (C6F5)SbCl2.2
HMPA XVII (C6F5)SbCl2.T

U
XVIII (C6F5)SbCl2.2
TU
175d 62 37.08(37
.10)
174 67 31.80(31
.83)
170 69 31.80(31
.83)
175 70 31.80(31
.83)
235 69 42.25(42
.27)
240 72 45.15(45
.18)
180 73 26.73(26
.74)
100 69 30.09(30
.11)
116 73 19.26(19
.29)
152 70 18.75(18
.77)
1.90(1.
95)
1.55(1.
56)
1.55(1.
56)
1.55(1.
56)
2.20(2.
22)
2.35(2.
37)
3.35(3.
37)
5.03(5.
05)
0.90(0.
93)
1.55(1.
58)
5.39(5.4
1)
3.05(3.0
9)
3.05(3.0
9)
3.05(3.0
9)
----
----
7.78(7.8
0)
11.65(11
.70)
6.42(6.4
3)
10.90(10
.94)

N)
I 357ms 290s 404, 604, 1568

II 460ms 310 404,604,1568(419,618,1585) V 458ms 289s 419, 621, 1610


IX 444ms 292s 880(840) XI 445ms 288s 1192(1130) XII 447ms 316s 1120
XIII 465 ms 292s 1212(1140)


XV 459 ms 315s 1212(1236) XVI 449 ms 290s 3300(3368) XVIII 452 ms 310s 3300(3380)
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Molecular adducts of pentafluorophenylantimony (III) chlorides (Rf) n SbCl3-n with neutral monodentate oxygen, nitrogen and sulphur donors 5
ISSN 2229-5518
Table 5 Relevant IR frequencies of
(pentafluorophenyl)antimony(III) derivatives.


R2SbCl RSbCl2 Assignments
220(ms) 221(w) d, t

290(w) 287(w) t
341(m) 339(ms) Sb-Cl

447(w) 451(vw) γ
560(m) 594(mw) p

605(m) 607(m) r
713(m) 715(m) s

779(m) 780(ms) ν
956(vs) 856(vvs) q s = strong; m = medium; w = weak; v = very
6 Conclusions
The replacement of an aryl group (C6H5, p-CH3C6H5) by pentafluorophenyl group enhances the Lewis acidity to such an extent as so to facilitate the formation of complexes. These complexes are monomeric and stable to atmospheric moisture and oxygen.
7 Acknowledgements
The authors are thankful to the Institute of Engineering and Technology, constituent college of GBTU, Sitapur Road, Lucknow, U.P., India for providing necessary laboratory facilities.
REFERENCES
[1] G. A. Fisher, N. C. Norman, Adv. Inorg. Chem. 41 (1994) 233-
271.
[2] M. Hall, D. B. Sowerby, J. Organomet. Chem. 347 (1988) 59-70; W. S. Sheldrick, C. Martin, Z. Naturforsch Teli B 46b (1991) 639.
[3] E. G. Zaitseva, S. V. Medevdev, L. A. Aslanov J. Struc. Chem. 31 (1990) 807-812.
[4] J. D. Smith In: Comprehensive Inorganic Chemistry, Pergamon
Press Oxford 2 , 1973 640.
[5] N. W. Alcock, M. Ravindran, S. M. Roe, G. R. Wiley, Inorg.
Chem. Acta. (1990) 167 115; N. W. Alcock, M. Ravindran, S. M. Roe, G. R. Wiley, Acta. Cryst. B49 (1993) 507-514.
[6] M. Nunn, M. J. Begley, D. B. Sowerby, Polyhedron 15 (1996)
3167-3174.
[7] J. L. Wardell Arsenic Antimony and Bismuth In: COMC I, 2 (1982) 681; COMC II 2 (1995) 341 F. G. A. Stone, G. Wilkinson, E. W. Abel, Eds. Pergamon Press, New York.
[8] Prem Raj, A. K. Aggarwal, A. K. Saxena J. Fluorine Chem. 42 (1989) 163-172.
[9] A. Oterio, P. Royo J. Organomet. Chem. 154 (1978) 13-19.
[10] B. A. Nevett, A. Perry, Spectrochim. Acta. A 31 (1975) 101-106. [11] Prem Raj, N. Misra, Ind. J. Chem. 30A (1991) 901-903.
[12] N. Nishii, Y. Matsumura, R. Okawara J. Organomet. Chem. 30
(1971) 59-65.
[13] R. G, Goel, H. S. Prasad, J. Organomet. Chem. 59 (1973) 253-257. [14] Firoz Zee, Ph. D. Thesis: Synthesis and characterisation of some new group V organometal derivatives and their reactions,
Lucknow University, Lucknow, India (1990).
[15] M. Hall, D. B. Sowerby J. Chem. Soc., Dalton Trans (1986) 1231-
1238.
[16] J. W. Dele, H. J. Emeleus, R. N. Haszeldine, J. M. Moss, J. Chem.
Soc. (1957) 3708.
IJSER © 2012
http://www.ijser.org



