International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 287
ISSN 2229-5518
Model for Analysis of Iron Extraction Based on As-Beneficiated Phosphorus Content and Iron Ore Grain Size Leached in H2O2 Solution
T. O. Chime
Department of Chemical Engineering, Enugu State University of Science & Technology, Enugu, Nigeria
Abstract
Quantitative analysis of iron extraction was carried out based on iron ore grain size and as-beneficiated phosphorus concentration during leaching in hydrogen peroxide solution. A model was derived and used as a tool for the analysis. The model is expressed as;
α = e- 0.0001 x + 42.5eθ + 5.0341
The validity of the two-factorial model was found to be rooted on the expression α - 5.0341 = e- 0.0001 x + 42.5eθ where both sides of the expression are correspondingly approximately equal. Statistical analysis of the extracted iron concentration as obtained from derived model and experiment for each value of the grain size considered shows standard errors of 2.74 x 10-5 and 0.4113% respectively. Deviational analysis indicates that the maximum deviation of the model-predicted iron extraction (from experimental results) is less than
2%. This translates to a confidence level above 98%.
Index Terms: Analysis, Iron Extraction, Grain size, Hydrogen Peroxide, Iron Ore
—————————— ——————————
1 INTRODUCTION
Hydrometallurgical extraction of metal from its ore through leaching in various solutions has been generally accepted to be significantly environmental friendly. There have been also increased research interests in exploring optimum methods of achieving balance between high yield and clean atmosphere. Intensive researches have been conducted on the dissolution of goethite in several inorganic acids belonging to the families of the carboxylic and diphosphoric acids in the presence of reducing agents (Chiarizia and Horwitz, 1991). A critical and comparative assessment evaluation carried out on the solubili- ty of iron in several organic and inorganic acids has shown that iron oxides and oxyhydroxides can dissolve in hydrochlo- ric and perchloric acids (Sidhu, et al.,1981). Investigations have been carried out of contact time, acid concentration, tempera- ture, particle size and, the stirring speed on the dissolution of the iron ore during a quantitative leaching of iron ore in hy- drochloric acid solution (Alafara et al.,2005). The dissolution rate was found to depend on the hydrogen ion concentration and temperature of the reaction system. The mechanism of dissolution appears to follow an exothermic pathway. The activation energy for the dissolution reaction was 13.63 kJmol-
1. About 92% of the total iron in the ore was dissolved within
120 min. by 12M HCl solution and 8000C using 0.1mm particle
size at an optimum stirring speed of 300rpm.
Analysis of results generated from these extraction process- es has been carried using various derived models which func- tioned as tools. A model for the evaluation of the concentra- tions of dissolved iron (relative to the final solution pH and temperature) during leaching of iron oxide ore in sulphuric acid solution has been derived (Nwoye et al., 2008). The model
%Fe = 0.35(α/T)3 (1)
depended on the values of the final pH and temperature of the
leaching solution which varied with leaching time. The posi- tive and negative deviations of the model-predicting values of
%Fe (dissolved) from those of the experimental values were found to be within the range of acceptable deviation limit for experimental results.
Calculations of the concentrations of leached iron during leaching of iron oxide ore in sulphuric acid solution has been achieved through application of a model (Nwoye et al., 2009a). The model is expressed as
%Fe = e-2.0421(lnT) (2)
The predicted concentrations of leached Fe were observed to be very close to the values obtained from the experiment. The model shows that the concentrations of leached Fe were dependent on the values of the final leaching solution temper- ature measured during the leaching process. It was observed that the validity of the model is rooted in the expression ln(%Fe) = N(InT) where both sides of the expression are corre- spondingly approximately equal.
A model was successfully derived for predictive analysis of the concentrations of dissolved iron during leaching of iron oxide ore in sulphuric acid solution (Nwoye et al.,2009b).The model expressed as
%Fe = 0.987(μ/T) (3)
was able to predict the concentrations of dissolved Fe with a high degree of precision. It was observed that the model was dependent on the values of the leaching temperature and weight of iron oxide ore added. The validity of the model was found to be rooted in the expression %Fe = N(μ/T) where both sides of the relationship are correspondingly approxi-
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 288
ISSN 2229-5518
mately equal. The maximum deviation of the model-predicted concentration of dissolved Fe from those of the experimental values was found to be less than 19% which is quite within the acceptable range of deviation limit for experimental results, hence depicting the usefulness of the model as a tool for pre- dictive analysis of the dissolved iron during the process.
A model for calculating the concentrations of dissolved iron during leaching of iron oxide ore in nitric acid solution was also derived (Nwoye and Ovri, 2010). It was observed that the validity of the model is rooted on the expression %Fe = N(μ/α) where both sides of the relationship are correspond- ingly approximately almost equal. The maximum deviation of the model-predicted dissolved %Fe values from the corre- sponding experimental values was found to be 28%. The mod- el

%Fe = 0.0043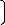 μ (4)
μ (4)
α
was found to be dependent on the value of the mass-input of
iron oxide ore and final solution pH measured during the
leaching process. Dissolved iron concentration per unit mass
of iron oxide ore input evaluated from experimental and mod-
el-predicted results were 0.0010%/g and 0.0011%/g respec- tively, indicating proximate agreement.
Also a model for calculating the concentrations of dissolved iron (relative to the final solution pH and temperature) during leaching of iron oxide ore in oxalic acid solution was derived (Nwoye and Mbuka, 2011) to evaluate the correlations be- tween dissolved iron & both final solution pH and tempera- ture. The model
%Fe = 1.1849(γ/T)3 (5)
was able to calculate the concentrations of dissolved iron being dependent on the values of the final leaching solution pH and temperature measured during the leaching process. It was observed that the validity of the model is rooted in the expression (%Fe/N)1/3 = γ/T where both sides of the expres- sion are approximately equal to 0.2. The maximum deviation of the model-predicted concentration of dissolved iron from the corresponding experimental values was found to be less than 18% which is quite within the acceptable range of devia- tion limit of experimental results. Concentrations of dissolved iron per unit rise in the solution temperature as obtained from experiment and derived model were evaluated as 0.0011 and
0.0015 %/0C respectively, indicating proximate agreement.
A model for predicting the concentration of iron dissolved
during nitric acid leaching of iron oxide ore in oxalic acid solu-
tion has been derived (Nwoye et.al., 2009c) to assess how the
final solution pH affects the extraction of iron. The model
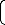
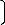
%Fe = 0.0133 α (6)
µ
was found to depend on the value of the final solution pH
and mass-input of iron oxide ore during the experiment. It
was observed that the validity of the model is rooted in the expression %Fe = N(α/μ), where both sides of the relationship are correspondingly approximately almost equal. Dissolved iron concentrations per unit mass of iron oxide ore input eval- uated from experimental and model-predicted results were
0.0058%/g and 0.006%/g respectively, indicating proximate
agreement.
Evaluation of the prospect and effectiveness of dissolving iron (from iron compounds) in organic acids such as acetic, oxalic formic, citric and ascorbic acids has shown that oxalic acid is most effective and promising because of its acid strength, good complexing characteristics and high reducing power, compared to other organic acids (Ambikadevi and Lalithambika, 2000).
Applicability of oxalic acid ensures precipitation of dis- solved iron from the leach solution as ferrous oxalate, which can be re-processed to form pure haematite by calcinations (Taxiarchour et al.,1997).
The aim of this work is to take a quantitative analysis of iron extraction based on iron ore grain size and as-beneficiated phosphorus concentration during leaching in hydrogen perox- ide. Phosphorus present in the iron ore is locked up with the ore and so during leaching of the iron ore, as phosphorus is being oxidized by oxygen (from hydrogen peroxide) and re- moved with time, iron is simultaneously being extracted.
MATERIALS AND METHODS
Agbaja (Nigeria) iron ore was mined and collected from the
deposit, beneficiated and the resultant concentrate used for
this research work. The iron ore was crushed for the purpose
of liberation size. Tyler standard was employed to produce
particle size of 250µm. The raw iron Agbaja iron ore was then
sent for chemical analysis using x-ray fluorescence diffraction spectrometer and atomic absorption spectrophotometer.
Scrubbing process
Scrubbing was carried to remove argillaceous materials
from the raw iron ore. The iron ore was poured into a head
pan and water was poured to a reasonable level. The ore was
washed and the water decanted. This was repeated for five
times until clear water was observed. At this point 5g of sodi-
um silicate and 25drops of oleic acid were sprinkled and dis-
tributed uniformly throughout the ore. 20litres of distilled wa-
ter was also introduced into the pan and the content mixed
thoroughly. After mixing, the argillaceous materials were re-
moved leaving behind the iron ore. The residue was washed thoroughly and was sun dried for 24hour. Some quantities were sent for chemical analysis.
Chemical leaching process
The dried scrubbed iron was further pulverized and sieved
to obtain particle sizes of 63, 90, 150, 180 and 250µm . Analar
grade of hydrogen peroxide solutions of different moles of 2,
4, 6, 8 and 10 were prepared. 50g of particle size of 63µm of
scrubbed iron ore was poured into a beaker(reactor). 10ml of
2M of hydrogen peroxide was poured into the beaker contain-
ing the iron ore. The mixture was thoroughly mixed to ensure
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 289
ISSN 2229-5518
homogeneity. The content was allowed to leach for 20, 40, 60,
80,and 100 minutes at 70ºC . At the end of each period, the
solution was cooled and filtered. The residue was collected,
washed to neutrality with distilled water, air dried and sun
dried at 150ºC for 24 hours. The experiment was repeated for
different concentrations and particle sizes. The samples were
analyzed using atomic absorption spectrophotometer and X
ray fluorescence diffraction spectrometer.
Model Formulation
Experimental data obtained from the highlighted research
work were used for the model derivation. Computational analysis of these data shown in Table 1, gave rise to Table 3 which indicate that;
α - K = e- S x + Neθ (7)
Introducing the values of K, S and N into equation (7)
α-5.0341=e-0.0001x+42.5eθ (8)
α = e- 0.0001 x + 42.5eθ + 5.0341 (9) Where
RESULTS AND DISCUSSIONS
The result of the chemical analysis carried out on the bene- ficiated iron ore concentrate is presented in Table 1. The table shows that the percentage of total Fe in the as-beneficiated ore is 52.67%.
Table 2: Result of chemical analysis of iron ore used
Model Validation
The validity of the model is strongly rooted in equation (8) (core model equation) where both sides of the equation are correspondingly approximately equal.Table 3 also agrees with equation (8) following the values of α - 5.0341 and e- 0.0001 x
+ 42.5eθ evaluated from the experimental results in Table 1.
Table 3: Variation of α - 5.0341 with e- 0.0001 x + 42.5eθ
(α) = Conc. of extracted iron (%)
(θ) = As-beneficiated phosphorus content (%)
x = Grain size (um)
K = 5.0341, S = 0.0001, N = 42.5
K, S and N are equalizing constant (determined using C-NIKBRAN (Nwoye, 2008))
Table 1: Variation of iron extracted concentration with leaching time and concentration of removed phosphorus
Furthermore, the derived model was validated by comparing the extracted iron concentration predicted by the model and that obtained from the experiment. This was done using vari- ous evaluative techniques such as statistical, graphical and deviational analysis.
76.4
76.2
76
75.8
Fe (%) | Grain size (µm) | (θ) (%) |
76.20 75.99 75.98 74.70 74.56 | 63 90 150 180 250 | 0.49 0.49 0.49 0.49 0.49 |
Boundary and Initial Condition
Consider iron ore (in a reactor) placed with in hydrogen
peroxide solution (oxidant).The reactor atmosphere is not con-
taminated i.e (free of unwanted gases and dusts). Initially,
75.6
75.4
75.2
75
74.8
74.6
74.4
R2 = 0.7955
0 100 200 300
Grain size (um)
atmospheric levels of oxygen are assumed just before the de- composition of H2O2 (due to air in the reactor). Mass of iron oxide ore: (50 g), leaching time considered: 40 mins., concen- tration of H2O2: 4M, constant treatment temperature: 70oC, ore grain size; 63µm, were also used.
The boundary conditions are: furnace oxygen atmosphere due to decomposition of H2O2 at the top and bottom of the ore particles interacting with the gas phase. At the bottom of the particles, a zero gradient for the gas scalar are assumed and also for the gas phase at the top of the particles. The re- duced iron is stationary. The sides of the particles are taken to be symmetries
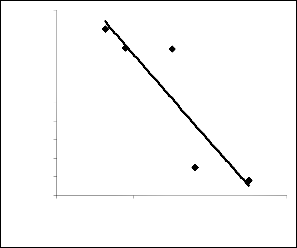
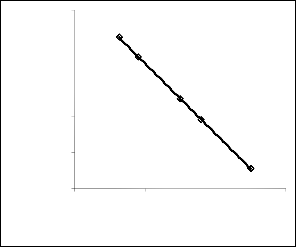
. 1: Coefficient of determination between extracted iron con- centration and ore grain size as obtained from experiment
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 290
ISSN 2229-5518
75.405 90
85
75.4
80
75.395 75
75.39 70
75.385
75.38
R2 = 1
0 100 200 300
Grain size (um)
ExD
65
MoD
60
0 100 200 300
Grain size (um)
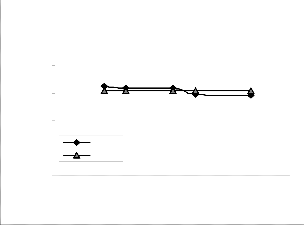
Fig. 2: Coefficient of determination between extracted iron concentration and ore grain size as obtained from derived
Statistical Analysis
Standard Error (STEYX)
Statistical analysis of the extracted iron concentration as ob- tained from derived model and experiment for each value of the grain size considered shows standard errors: 2.74 x 10-5 and 0.4113% respectively. The standard error was evaluated using Microsoft Excel version 2003.
Also the correlations between extracted iron concentration
and grain size as obtained from experiment and derived mod-
el considering the coefficient of determination R2 from Figs.
1and 2 was calculated using the equation;
R = √R2 (16)
The evaluations show correlations 0.8919 and 1.0000 respec-
tively. These evaluated results indicate that the derived model
predictions are significantly reliable and hence valid consider-
ing its proximate agreement with results from actual experi-
ment.
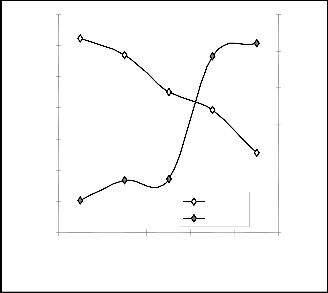
Graphical Analysis
Comparative graphical analysis of Fig. 5 shows very close alignment of the curves from model-predicted extracted iron concentration (MoD) and that of the experiment (ExD). The degree of alignment of these curves is indicative of the proxi- mate agreement between both experimental and model- predicted extracted iron concentration
Fig. 3: Comparison of the extracted iron concentration (rela- tive to ore grain size) as obtained from experiment and derived model
Deviational Analysis
Analysis of extracted iron concentrations from the experiment and derived model revealed deviations on the part of the model-predicted values relative to values obtained from the experiment. This is attributed to the fact that the surface prop- erties of the iron ore and the physiochemical interactions be- tween the ore and the oxidant (H2O2) which were found to have played vital roles during the process were not considered during the model formulation. This necessitated the introduc- tion of correction factor, to bring the model-predicted extract- ed iron concentration to those of the corresponding experi- mental values.
Deviation (Dn) of model-predicted removed phosphorus con- centration from that of the experiment is given by

Dn = Pv –Ev x 100 (17) Ev
Where
Pv = Extracted iron concentration as predicted by de-
rived model
Ev = Extracted iron concentration as obtained from ex-
periment

Correction factor (Cr ) is the negative of the deviation i.e Cr = -Dn (18) Therefore
Cr = - Pv – Ev x 100 (19) Ev
Introduction of the corresponding values of Cr from equation (19) into the derived model gives exactly the extracted iron concentration as obtained from experiment.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 291
ISSN 2229-5518
75.405
75.4
75.395
75.39
75.385
75.38
75.375
75.37
Model
Deviation
63 90 150 180 250
Grain size (um)
1.5
1
0.5
0
-0.5
-1
-1.5
Comparative analysis of Figs. 6 and 7 indicates that the orien- tation of the curve in Figs. 7 is opposite that of the deviation of model-predicted extracted iron concentration (Fig. 6). This is because correction factor is the negative of the deviation as shown in equations (18) and (19).
It is believed that the correction factor takes care of the effects of surface properties of the iron ore and the physiochemical interactions between the ore and the oxidant (H2O2) which have played vital roles during the process, but were not con- sidered during the model formulation. Fig. 7 indicates that the least and highest magnitudes of correction factor to the model- predicted extracted iron concentration (from the correspond- ing experimental values) are + 0.77 and - 1.1 % which corre- sponds to extracted iron concentrations: 75.3926 and 75.3828
%, as well as grain sizes: 150 and 250 um respectively.
It is important to state that the deviation of model predicted results from that of the experiment is just the magnitude of the value. The associated sign preceding the value signifies that
Fig. 6: Variation of model-predicted extracted iron concentra- tion with associated deviation from experimental results (rela- tive to ore grain size)
Figs. 6 and 7 show that the maximum deviation of the model-predicted extracted iron concentration from the corre- sponding experimental values is less than 2% and quite within the acceptable deviation limit of experimental results. The fig- ure show that the least and highest magnitudes of deviation of the model-predicted extracted iron concentration (from the corresponding experimental values) are – 0.77 and + 1.1 % which corresponds to extracted iron concentrations: 75.3926 and 75.3828 %, as well as grain sizes: 150 and 250 um respec- tively
the deviation is a deficit (negative sign) or surplus (positive sign).
CONCLUSIONS
Quantitative analysis of iron extraction was carried out based on iron ore grain size and as-beneficiated phosphorus concen- tration during leaching in hydrogen peroxide solution. A model was derived and used as a tool for the analysis. The validity of the two-factorial model was found to be rooted on the expression α - 5.0341 = e- 0.0001 x + 42.5eθ where both sides of the expression are correspondingly approximately equal. Statistical analysis of the extracted iron concentration as obtained from derived model and experiment for each value of the grain size considered shows standard errors of 2.74 x 10-
5 and 0.4113% respectively. Deviational analysis indicates that
75.405
75.4
75.395
75.39
75.385
75.38
75.375
75.37
Model
Corrfactor
63 90 150 180 250
Grain size (um)
1.5
1
0.5
0
-0.5
-1
-1.5
the maximum deviation of the model-predicted iron extraction (from experimental results) is less than 2%. This translates to a confidence level above 98%.
REFERENCES
[1] Alafara, A. Baba, D., Adekola, F. A., Folashade, A. O. (2005). Quantitative Leaching of a Nigerian Iron Ore in Hy- drochloric Acid, J. Appl. Sci. Environ. Mgt. 9(3):15 - 20
[2] Ambikadevi, V. R.., Lalithambika, M. (2000). Effects of Or- ganic Acids on Ferric Iron Removal from Iron-Stained Kaolin- ite. Applied Clay Science, 16:133-145.
[3] Chiarizia, R., Horwitz, E. P. (1991). New Formulations of
Iron Oxides Dissolution. Hydrometallurgy, 27:339-3
[4] Nwoye, C. I., Obasi, G. C., Mark, U., Inyama, S., Nwakwuo,
C. C. (2009a). Model for Calculating the Concentration of
Leached Iron Relative to the Final Solution Temperature dur-
ing Sulphuric Acid Leaching of Iron Oxide Ore. New York
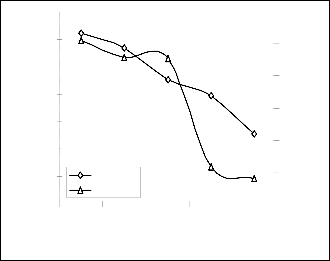
Fig. 7: Variation of model-predicted extracted iron concentra-
tion with associated deviation from experimental results (rela-
tive to ore grain size)
Science Journal, 2(3):49-54.
[5] Nwoye, C. I. (2008). C-NIKBRAN: Data Analytical Memory
[6] Nwoye, C. I., Amara, G. N., and Onyemaobi, O. O. (2008).
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 292
ISSN 2229-5518
Model for Evaluating Dissolved Iron during Leaching of Iron
Oxide Ore in Sulphuric Acid Solution, Inter. J. Nat. Appl. Sc.,
4(2): 209- 211.
[7] Nwoye, C. I., Ofoegbu, S. U., Obi, M. C., Nwakwuo, C. C.
(2009b). Model for Predictive Analysis of the Concentration of
Dissolved Iron Relative to the Weight Input of Iron Oxide Ore
and Leaching Temperature during Sulphuric Acid Leaching.
Nature and Science Journal. 7(3):41-47.
[8] Nwoye, C. I. and Ovri, J. E.O. (2010). Model for Calculat-
ing the Concentration of Dissolved Iron Relative to the weight-
input of Iron Oxide Ore and Final Solution pH during Nitric
acid leaching, Journal of Engineering Science and Technology,
5(2):151-164
[9] Nwoye, C. I., Nwobodo, C. S., Nlebedim, C., Nwoye, U. C.,
Umana, R., and G. C. Obasi (2009c).
[10] Model for Predicting the Concentration of Iron Dissolved during Nitric Acid Leaching of Iron Oxide Ore in Oxalic Acid Solution, New York Science Journal, 2(6):1-12.
[11] Nwoye, C. I., Mbuka, I. E. (2011). Model for Calculating the Concentration of Dissolved Iron Relative to the Final Solu- tion pH and Temperature during Oxalic Acid Leaching of Iron Oxide Ore. Journal of American Science, 7(1):12-18.
[12] Sidhu, P. S., Gilkes, R. J., Cronell, R. M., Posner, A. M., Quirk, J. P. (1981). Dissolution of Iron Oxides and Oxydrox- ides in Hydrochloric and Perchloric Acids. Clays Clay Miner- als, 29:269-276.
[13] Taxiarchour, M., Panias, D., Doumi, I., Paspaliaris, I., Kon- topoulos, A. (1997). Removal of Iron from Silica Sand by Leaching with Oxalic Acid, Hydrometallurgy, 46: 215-227.
IJSER © 2014 http://www.ijser.org




