International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1114
ISSN 2229-5518
MÖSSBAUER SPECTROSCOTIC STUDY OF CHEMICAL STATE OF IRON IN NATURAL
SAMPLES HORNBLENDES FROM UDAIPUR
DISTRIC (RAJ)
ROHITASH KUMAR, *H.S.SINGH
Abstract— Mössbauer spectroscopy is based on the quantum mechanical “Mössbauer effect,” which provides a non-intuitive link between nuclear and solid-state physics. Mössbauer spectrometry provides unique measurements of electronic, magnetic, and structural properties within materials. Mössbauer study of hornblendes samples are reported here with Mossbauer parameters and also chemical analysis. The state of iron present in the form of Fe2+and Fe3+ cations in the samples. The ratio of Fe2+ and Fe3+ leads to oxidation state of iron oxides which is related to F-factor.
Index Terms— Mössbauer study, hornblendes samples, Oxidation state.
—————————— ——————————
1 INTRODUCTION
OST applications of Mössbauer spectroscopy in materi- als science utilize “hyperfine interactions,” in which the electrons around a nucleus perturb the energies of nu-
clear states. The energy levels of a nucleus are determined
mostly by the strong interaction which binds the nucleons
together. Mössbauer spectrometry is performed with the nu-
clei 57Fe. The 57Fe atoms in the lattice oscillate at higher fre- quencies in at higher temperatures, leading to an energy shift of the accompanying absorption peak from the Doppler Effect. Mössbauer spectroscopy has been widely used to investigate the chemical state of iron in various kinds of hosts in metals [1], [2]. Among the various forms of iron oxides maghemite and hematite are of most importance in technology and indus- trial applications [3]. Maghemite has numerous application like recording, memory devices, magnetic resonance imaging, drug delivery or cell targeting [4] (Laurent et al., 2008; Daou et al., 2010).
The octahedral coordinated with four oxygen and two hy- droxyl ions. Now our aspect has been of major interest. The change in oxidation state with respect to nucleus [5], [6], [7]. The change of spin state with respect to the electronic struc- ture of the iron atom obtained from corresponding Mössbauer experiment [8], [9], [10] and nature of neighbouring atoms and two isomers are distinguished which are either C is form of Trans form [11], [12], [13], [14]. The basic structure feature of
————————————————
• Rohitash kumar is an Research Scholar with The Department of Physics
Faculty of Science, Jai Narain Vyas University, Jodhpur. India, Phone;
+91-9461056793; E-mail: rohit_mehariya@yahoo.com
• H.S.Singh is an Associate Professor with The Department of Physics Fac- ulty of Science, Jai Narain Vyas University, Jodhpur. India, Phone; +91-
9460530598; E-mail: harisaransingh1961@gmail.com
silicate hornblendes is a composite of different site octahedral sites are generally fitted by Mg2+, Fe2+, Fe3+, Al3+, Mm2+ etc. There are two types of octahedral sites present in silicate de- pending on the position of the hydroxyl ions. If the hydroxyl ions on the adjacent corner of the site it is known as the C’s site. While the corresponding site trans site describe the site situation where hydroxyl ions lie at the opposite corner of the octahedral [15], [16], [18], [19].
The samples were collected of medium grade metamorphism
Udaipur district (Raj). Tectonic process, which occur in a high-
er pressure, high temp environment change the composition
and structure of original igneous and sedimentary rocks. Pure
fraction of the samples, were obtained from by using gravity magnetic and microscopic separation methods. These samples are ninety-nine percent pure.
2 EXPERIMENTAL METHOD
The hornblendes samples were collected from geology de- partment and Chemical analysis carried out from chemistry department J.N.V University Jodhpur. The Mössbauer spec- trometer consist of a electromechanical drive in a constant ac- celeration mode a proportional counter with accessory elec- tronic backed by a channel analyzer, due to the symmetrical
256 channels and mirror images of each other are recorded
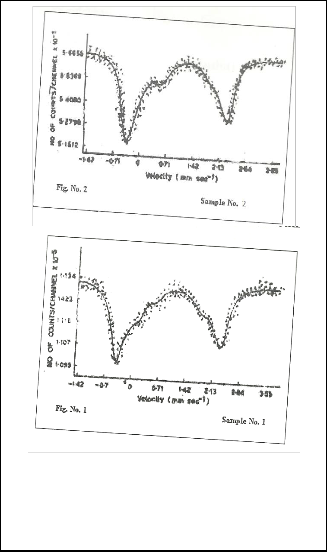
simultaneously two typical spectra is displaced in figures
1and 2. The source is mounted on the drive unit which was
10m CiCo57 diffuse in to palladium and spectra were recorded
in transmission geometry at room temperature the spectra have been analyzed by computer programme. This pro- gramme assumes the spectrum to be sum of Lorentzian. We have restricted these fits to maximum of 8 lines corresponding to 4 doubles, ferrous and ferric ions in sites.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1115
ISSN 2229-5518
TABLE 1
3 RESULT AND DISSCUSSION
3.1 Figures and Table
CHEMICAL ANALYSIS OF SOME HORNBLENDES
Sample S-1 S-2
SiO2 45.44 44.90
TiO2 01.48 00.87
Al2 O3 09.73 12.42
Fe2 O3 05.84 07.27
FeO 05.88 09.97
MnO 00.12 00.43
MgO 12.50 12.79
CaO 09.49 07.89
Na2 O 01.12 01.67
K2 O 00.39 00.67
P2 O5 00.31 00.31
TABLE 2
CHEMICAL COMPOSITION CALCULATION ON THE BASISIS OF OX- YGEN PER FORMULA
SAMPLE S-1 S-2
Si | 6.7369 | 6.4467 |
Ti | 0.1637 | 0.0924 |
Al3+ | 0.4372 | 0.5481 |
Fe2+ | 1.2246 | 1.2051 |
Fe3+ | 0.6500 | 0.7127 |
Mn2+ | 0.0142 | 0.0509 |
Mg | 2.7384 | 2.8091 |
Cu2+ | 1.5131 | 1.2166 |
Na+ | 0.2939 | 0.4631 |
K+ | 0.0712 | 0.1243 |
Fig. 1and 2 Show that.the Mossbauer Spectra of Hornblendes
Samples at Room Temprature.
TABLE3
EXPERIMENTAL RESULT OF MOSSBAUER PARAMETERS
SAMPLE IS QS LW F-FACTOR ASSIGNMENT
The chemical analysis of samples indicate that the amount of iron oxide is smaller than SiO2 but Mössbauer spectroscopy can be used to identify iron species because this technique can quantify the proportion of Fe2+ and Fe3+ in both crystalline and amorphous materials [20], [21]. So iron species is identified as ferric iron of hematite (Fe3+), para magnetic ferric iron (Fe3+) and para magnetic ferrous iron (Fe2+). But Fe2+ is the paramag- netic spices present in the octahedral sheet of hornblendes. The chemical analysis revels that the amount of iron is less but which is useful for Mossbauer spectroscopy.
Errors in IS ± 0.02% QS ± 0.018% LW ± 0.02%
The Mössbauer lines are fitted in the form of doublets. The isomer shift is varied from 0.39mm/sec to 1.17mm/sec. The isomer are shift depend on electron density at the nucleus. If electron in outermost orbit involves in chemical bonding have a significant perturbing effect on occupied 3s orbital and σ bonds 4s orbital. The isomer shift parameter is sensitive to any
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1116
ISSN 2229-5518
factor that effect the number and distribution of valence shell electron it provide a iron oxidation state, coordination envi- ronment and covalent character of bonded anions. Since Fe3+ is a low spin cation. So the Fe2+ cations no longer retains and electron d6 orbital of Fe, therefore decrease and electron densi- ty at the nucleus produces relatively high isomer shift. The doublet with a smaller quadrupole splitting is ascribed to para magnetic ferric (Fe3+), presumely originating from clay miner- als. The doublet with a larger quadruple (QS) is attributive paramagnetic ferrous (Fe2+) mostly due to crystalline silicate [22]. When ferrous Fe is bounded to oxygen in silicate, sul- phate carbonates increase positive charge of Fe3+ion formed by losing one of its 3d electron and remaining electrons are at- tracted by nucleus isomer shift ferric mineral are smaller than the ferrous mineral. The covalent bond character is higher tet- rahedral coordination than for a cation in a octahedral coordi- nation with O or S. As a result decreased isomer shift correlat- ed with lowering of coordination number of the Fe cation. Non of samples represent the quadrupole splitting zero and Fe nucleus is not spherical in hornblendes. The quardupole split- ting is varied from 0.53mm/sec to 2.84 m/sec. This represent that Fe nucleus are not spherical in the samples, a non spheri- cal component of the electric field. The QS value of Fe2+ ion is larger than the QS value of Fe3+ion. This variation indicates that the coordinate sites are destroyed in geometry of horn- blendes. Therefore as aresult in electronic configuration that is no longer spherically symmetrical and produces an electric field gradient at the nucleus. So QS parameters are significant- ly higher for Fe2+ bearing silicate or oxides than for those min- erals containing Fe3+ ions. Thus Fe2+ and Fe3+ions in horn- blendes are to be easily distinguished by Mössbauer spectros- copy. Octahedral coordinated Fe in the majority of rock form- ing minral in distinguished from Fe2+ion while Fe3+ ions occu- py very distorted and noncubic lattice environment around the nucleus. Mössbauer spectroscopy is useful technique for characterizing the valence electronic coordination symmetric and state of occupancies of Fe cations in rock forming miner- als. There are two main parameters isomer shift quadrupole splitting and to distinguished Fe2+ and Fe3+ ions with sites. Peak area is related to recoil free fraction.
4 CONCLUSION
Our main conclusions are as follows.
1- The chemical analysis of both samples represent that the amount of iron oxide is very small.
2- Both samples are indicating that the iron present in
the form of ferrous (Fe2+) and the range of QS is 1.83 to
2.83. it means that ferrous is in low spin state.
3- The ratio of Fe3+/Fe2+ is more than 50%. This show that
Fe2+ is in low spin state.
4- Mica- Fe is not a reasonable ferric/ ferrous iron stand-
ard since it revels significant variations in degree of oxidation with different size fractions are .1 to .5.
5- The QS value of Fe2+is large than the QS values of Fe3+ this variation represent that coordinates sites are de- stroyed in geometry in hornblendes. So Fe3+ is not
high spin state.
ACKNOWLEDGMENT
I wish to thanks all staff to supported me for this work.
REFERENCES
[1] H. Annersten, Amer mineralogist. Mössbauer studies of natural bio- tites. Vol. 59, pp. 143 (1947).
[2] M.Blume., Phys, Rev, Letter. Hyperfine Structure and Nuclear Radia- tion, Vol. 14, pp. 506 (1965).
[3] Huo L, Li W, Lu L., Cui H., Xi S., Wang J., Zhao B., Shen Y., and Lu Z., Preparation, structure, and properties of three dimensional or- dered α-Fe 2 O3 nanoparticulate film. Chem. Mater. Vol. 12, No. 3, pp.
790 (2000).
[4] Daou T. J., Greneche J-M., Lee S-J., Lee S., Lefevere C., Sylvie B-C., and Pourroy G., Spin canting of maghemite ostudied byu NMR and In-Field Mossbauer spectrometry. J. Phy. Chem. C Vol. 114, No. 19, pp. 8794-8799 (2010).
[5] J. P. Adloff, J. M. Friedt, Pruc, Symp, Mössbauer spectroscopy, apl
IAEA. 30 (1972).
[6] H. H. Wickman, G. K. Wertin, in Chemical application of Mössbau- er.
[7] J. Ensling, B. W Fitzsimmons, P. Gutlich., K.M. Hasselback, Ang, chem., 82 638 (15) (1970).
[8] J. English, J. Fleisch, P. Gutleich, B. W. Fitzsimmons, chem. Lett, 45,
22 (1977).
[9] R. Chandra, R. P. Tripathi and S.lokanthan, Phy. Satat. 88, 633 (1978). [10] T. S. Srivastava, A. Nath, Radiochem radioanal Letters 16, 103 (1974). [11] R. V. Parish, in Ernst A. Koerner von Gustorf , The organic chemis-
try of iron, 2 vols (Academic press, New York, P 175 (1978). [12] R. V. Parish, Prog Inorg chem., 15-101, (1972).
[13] G.K. shenoy and F.E. Wagner, Mössbauer isomer shifts, (North Hol- land, Amsterdam (1978).
[14] Banchraft G. M Maddock A. G and Burns R. G Geo Chem, Acta 31,
2219-2246 (1967).
[15] Tripathi, R.P. Chandra U. Candra R. And Loknathan S. J. Inorg, Nucl
(1978).
[16] Haggstron L. Wappling R. And Annersten H. Phys. Stat Sol, 33, 741 (1969).
[17] Greava C Burns R, G and Barcott, GMNature 61, 229 (1971).
[18] A.N Nigam, R.P. Tripathi, H.S. Singh and R.S. Gambhir Indian Jour- nal of pure and applied physics Vol 25, 188-190, April (1987).
[19] A.N Nigam, R.P. Tripathi, H.S. Singh and R.S. Gambhir Fuel (GB),
68, 209 (1989).
[20] A.N Nigam, R.p. Tripathi, H.S. Singh and R.S. Gambhir Indian Fuel
(GB) 70, 262 (1981).
[21] J. HILTON, G.J. Long, J.S. CHAPMAN, J.P.LISHMAN, Geo- chim.Coschin. Acta, 50, 2147 (1986).
[22] J.M.D. Coey, D.W. Schindler, F. Weber, Can.J. Earth Sci, 11, 1489 (1974).
IJSER © 2014 http://www.ijser.org
