INTRODUCTION
C
ytochrome P450 and NO synthase (NOS) among heme enzymes have strong oxidizing ability and unusual struc- ture, in that their heme irons have thiolate coordination
[1-6]. Nitric oxide synthase, unusual members of the cyto-
chrome P-450 family, catalyze the five-electron oxidation of L-
arginine to nitric oxide (.NO) and citrulline at the expense of NADPH and O2 [7,8]. Nitric oxide a messenger molecule, par-
ticipates in important physiological processes, such as vasodi- latation [9], neuronal signal transmission [10], cytotoxicity against pathogens and tumors [11], and cellular respiration activity [12]. Metalloporphryrins are generally involved to prepare artificial mimics of heme proteins. Attempts to model Nitric oxide synthase using iron (III) porphyrins [13,14] suffer from some lacunae, most of them ignored L-arginine as the starting substrate for their models. There is inadequate infor- mation concerning the direct oxidation of L-arginine with hy- drogen peroxide in the presence of stable thilote-ligated iron porphyrin as catalyst. We have synthesized the synthetic heme thiolate (SR complexes) which retains thiolate coordination during catalytic oxidation of L-arginine (Scheme I). Organic peroxide, or more rarely environmental friendly oxidant H2 O2 are known to be a substitute for NADPH and O2 in this cata- lytic cycle [15]. The introduction of ferryl group from Fe+3 in this complex requires the treatment of catalyst with hydrogen peroxide. Therefore the idea of the shunt mechanism was opt- ed by using peroxide, to carry out these oxidation reactions.
EXPERIMENTAL
aterials
All chemicals and reagents used in this study were of analyti- cal grade and procured from Sigma Aldrich, Fluka and Spec- trochem Pvt. Ltd. Organic solvents were freshly distilled be- fore use. Deuterated solvents were purchased from Aldrich and used without purification. Distilled water was obtained
from Milli-Q water purification system (Millipore). Griess rea- gent used in the reactivity study was prepared freshly by mix- ing aliquots of 1% sulphanilamide in 4 N HCl and 0.2 ml of 0.1% N-(1-naphthyl) ethylenediamine in 0.4 N HCl and their absorption was measured at 543nm.
hysical Measurements
IR spectra were recorded on (5-Dx) Nicolet FTIR instrument with KBr pellets. NMR spectral characterization was carried out with a Bruker DPX 300 MHz instrument with tetrame- thylsilane as internal standard for chemical shift measure- ments. UV-Vis spectral measurements were taken with a Per- kin-Elmer λ2S spectrophotometer interfaced with a 486 digital computer. The cell holder of the spectrophotometer was con- nected to a Julabo F-30 temperature regulator. Absorbances against time were recorded by using PECSS software devel- oped by Perkin Elmer. X-ray diffraction patterns of powdered samples were obtained, using Bruker D8 advanced diffrac- tometer equipped with a rotating anode and Cu Kα radiation. HPLC measurements were done by reverse phase HPLC, of o- phthalaldehyde (OPA) derivatives with SF 970 fluorescence detector equipped with a deuterium lamp. The OPA deriva- tives were detected with a monochromator set at around 330nm(excitation wavelength) and around 418nm(emission wavelength) cut-off filter. Column used was Ultrasphere ODS 25cm X 4.6mm I.D., 5mm particle size.
Catalyst Preparation
Condensation of pyrrole and four equivalents of o- nitrobenzaldehyde in acetic acid gave meso-tetra(o- nitrophenyl)porphyrin which was reduced by stannous chlo- ride to the meso-tetra(o-aminophenyl)porphyrin (Scheme I). The four atropisomers were separated by chromatography, the slowest moving of which was the desired isomer A shown in the scheme I.
————————————————
Dr. Monalisa Mukherjee is Assistant Professor in the Amity Institute of Biotechnology, Amity University, India. Mobile +91-9873279964. E-mail: mmukherjee@amity.edu
IJSER © 2013

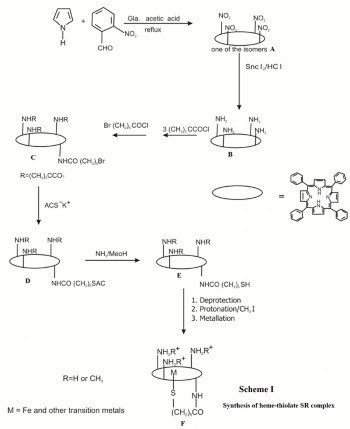
Interconversion of the atropisomers was sufficiently slow at room temperature to afford clean separation. Refluxing the unwanted products in toluene for 20 min effected reequilibra- tion to the statistical mixture allowing further isolation of the desired isomer. Reaction of the amino groups with pivaloyl chloride( trimethylacetyl chloride) gave the "picket-fence" porphyrin in which the configuration is frozen by the bulky substituents. Reaction with omega bromo acetoyl chloride gives the compound C, which on treatment with potassium salt of thio-acetic acid gives the compound D. D further reacts with ammonia in presence methanol to give compound E. Fi- nally compound E undergoes de-protection, followed by pro- tonation in presence of methyl iodide and metallation with iron bromide to give desired SR complex F. Since the S- protected pentanoic acid derivatives were more difficult to obtain, the bromoalkyl chain was first attached to the porphy- rin to give C and the thio group was introduced by treatment with ACS-K+. Deacetylation (MeOH/NH3 ) gave the free thiol. The reaction of compound B with excess isonicotinic chloride hydrochloride yielded the tetra-isonicotinamide TPP as a sta- tistical mixture of atropisomers which could be separated by chromatography. The isonicotinamide groups were then methylated with methyl iodide, followed by anion exchange, to yield the water soluble tetrakis-(N- methylisonicotinamidophenyl) porphyrin-tetracation. This compound can undergo remaining steps to yield water soluble heme-thiolate complex as shown in Scheme I. SR-Imidazole complex was prepared as per literature procedure [16].
Two anionic porphyrins FeTPPS and FeTCP and two cationic
porphyrins FeTPAP and FeTMPyP were synthesized with modified literature procedure [17-21]. These ionic porphyrin complexes were loaded on counter ionic dowex resin. Imidaz- ole covalently bound to iron porphyrin are synthesized and investigated by literature procedures [22-23].
Catalysis
The The nonthiolated and thiolated porphyrins (20 mg) was taken in a cuvette and 1 ml of L-arginine (50 mM) was added with a pipette. The reaction was initiated by adding 0.5 ml of H2 O2 (100 mM) and continued for 20min at 25oC. NO2 − formation was determined by an automated procedure based on Griess reaction. The contents were shaken for a minute and the absorbance at 543 nm was noted. Nitrite standard refer- ence curve was prepared in water in high concentration range as well as in low concentration range. The average absorbance value of each concentration of nitrite standard was plotted against nitrite concentration. The amount of (NO2 -) released was determined from the nitrite standard reference curve. Amino acid product was identified using two different tech- niques.
RP-HPLC of OPA derivative. The derivatization procedure
was followed as described by Umagat et al. [24]. The product was analyzed with good selectivity and sensitivity by re- versed-phase high-performance liquid chromatography. 10ml of the derivatized sample was injected onto the column. The mobile phase was linear step gradient with a ternary solvent mixture, THF/ 0.05mol/L sodium acetate, pH 6.6/ methanol. The flow rate for this method was 1.5ml/min. Amino acid standards were used to quantify samples. 2-aminoethanol was
used as an internal standard. Detection was generally possible in picomole range.
The formation of amino acid was also identified using NMR
probe. NMR spectra of L-arginine and L-citrulline procured from the market were recorded in D2 O in presence of SR com- plex. The spectra are shown in fig.1a &1b. 20mg of axial thio-
lated water-soluble porphyrin was used for these experiments because of the limitation in NMR tube. 5mg of L-arginine dis- solved in 0.6 ml D2 O was added. Finally 6.4 ml of aqueous 30% H2 O2 was added to initiate the reaction. The contents of the tube were shaken for taking the NMR spectrum.
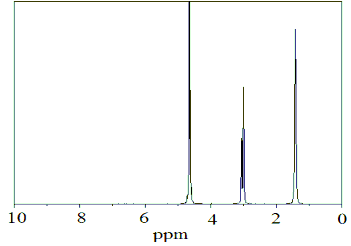
Fig.1a. NMR spectra of L-arginine(5mg) + heme-thiolate com- plex (20mg) + D2 O(0.6ml)
2
Formation of nitrite (NO −) as a function of hydrogen peroxide concentration in the oxidation of L-arginine with SR complex
The nonthiolated and thiolated porphyrins oxidation reactions of L-arginine were carried out at 25oC by varying the H2 O2 concentration using heme-thiolated (SR complex). L-arginine (35 mg, 200 mM) in 1ml deionized water and SR-complex (20 mg, 0.2752 mmol axial thiolated porphyrin, SR complex) were taken in a vial pre-occupied with a magnetic stir-bar. In each experiment after the addition of aqueous 30% H2 O2 , the reac- tion mixture was stirred for 5-6 min. Finally the reaction mix- ture was transferred to the cuvette containing Griess reagent. The cuvette was shaken for a minute and the absorbance was measured at 543 nm. From the calibration plot of nitrite, the concentration of nitrite corresponding to measured absorbance value was obtained.
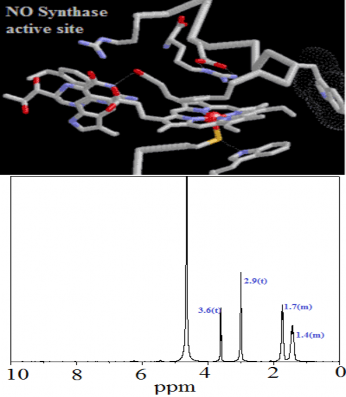
Fig.1b. NMR spectra of L-arginine(5mg) + 30% aqueous H2 O2 (6.4µl) + D2 O(0.6ml) + Heme-thiolate complex (20mg)
Stoichimetry of L-arginine consumption and product formation
The reaction mixture contains: 38.7 mM H2 O2 , 200 mM L- arginine in 1ml deionized water and heme-thiolate SR com- plex (20-50mg). After addition of aqueous 30% H2 O2 , the reac- tion was continued for 20 min at 25oC. Finally 2 ml of Griess reagent (freshly prepared) was added to it. The contents were shaken for a minute and the absorbance at 543 nm was noted. Nitrite standard reference curve was prepared in water in high concentration range as well as in low concentration range. The average absorbance value of each concentration of nitrite standard was plotted against nitrite concentration. The amount of (NO2 -) released was determined from the nitrite standard reference curve. Amino acid product was analyzed as described in section 2.4. Concentration of L-arginine and Citrulline were determined from their standard curves (con- centration vs. peak area) using HPLC methodology.
RESULT AND DISCUSSION
The most successful of the heme protein active site models is the "picket-fence" porphyrin. Steric encumbrance about the metal site of these substituted TPP( tetra phenyl porphyrin) molecules depends on two factors:
due to steric repulsion, the TPP will adopt a conformation
in which the four meso- phenyl rings are essentially perpen-
dicular to the porphyrin ring; substituents at the orr/io-
positions of the phenyl rings will lie above and below the por-
phyrin plane, and
for TPP molecules containing mono-ortho-substituted phenyl rings, separation and, depending on the bulk of the subsituent, interconversion of the four possible atropisomers may be achieved. The synthesis of a substituted iron(II) tetra- phenylporphyrin having four pivalamido groups located on the same side of the porphyrin ring would give a "protected pocket". Ligands e.g. imidazole, could bind to the metal on the open face but could not penetrate the pocket, thereby ensuring five-coordination even in the presence of excess ligand. The formation of amino acid (citrulline) and inorganic product (NO2 − ) from L-arginine and H2 O2 was determined as a func- tion of the amount of the SR catalyst. Each sample was ana- lyzed separately for citrulline and nitrite. Amount of NO re- leased from L-arginine with H2 O2 using the ctalysts were monitored spectrophotometrically at 543 nm with time. Griess reagent reacts with NO2 − to produce a chromophore which absorbs at 543nm. The formation of NO2 − produced from L- arginine and H2 O2 was observed with time at 25oC for the thi- olated and nonthiolated catalyst (data not shown). The result showed that the release of nitric oxide was instantaneous, when thiolated iron porphyrin was used. The reaction with iron porphyrins coordinated with chloride or imidazole is very slow process compared to the thiolated one. Most likely, hydrogen peroxide reacts with the catalyst to form high valent iron oxo intermediate, which contains ferryl group [Fe=O]+3 , responsible for the oxidation of L-arginine. The dependence of formation of NO2 − on the concentration of hydrogen peroxide was determined for the heme-thiolate SR complex catalyzed oxidation of L-arginine at 25OC. All reactions were continued for six minutes in order to collect data for each concentration. The product formation was linear with time over this interval and after that deviates from linearity. The results of these reac- tions are shown in fig.2. The data presented were average of duplicate set of experiments. This study was done to deter- mine the kinetics (or kinetic parameters) of H2 O2 dependent oxidation of L-arginine.
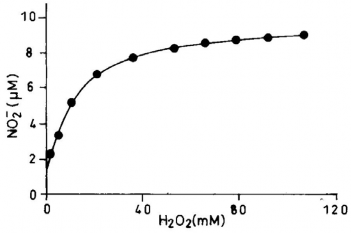
Fig.2. Formation of nitrite (NO2−) as a function of hydrogen peroxide concentration in the oxidation of L-arginine with heme-thiolate (SR complex)
The formation of citrulline and NO2 − were stoichiometric to the amount of L-arginine consumed. Within experimental er- ror, for every mol of arginine consumed, one mol each of cit- rulline and NO2 − were formed shown in Table 1. The amino acid product of the reaction was shown to be L-citrulline. L- arginine and L-citrulline were eluted at 23.7 min and 21.4 minutes respectively. 2-aminoethanol (internal standard) was eluted at 32 minutes. No evidence was suggestive of the for- mation of any other amino acid product. Chromatographic identification of citrulline was made by reverse-phase HPCL of OPA derivative and agreed with the retention time (fig.3) of identically treated citrulline standard. Unequivocal evidence of citrulline formation was also obtained from NMR spectros- copy. Identification of citrulline can be readily made by moni- toring its peak at 3.6 (t), 2.9(t), 1.74-1.66(m) and 1.4 – 1.3(m), which clearly distinguishes its presence even in presence of L- arginine.
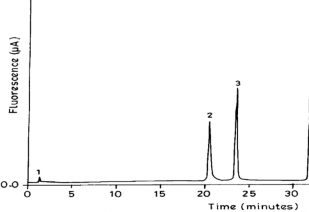
Fig.3. Elution profile of OPA-derivatized amino acid product from oxidation of L-arginine of H2 O2 dependent reaction catalyzed by heme-thiolate SR complex.
No evidence was to support for the formation Nδ-hydroxy-L- arginine (L-NHA) in this oxidation reaction. This may be due to the instability of L-NHA in presence of excess peroxide in the reaction mixture. The activity of the catalyst was indicated by the degree of L-arginine conversion. SR complex showed better performance than the iron porphyrin coordinated by chloride or imidazole. The yields of L-citrulline and NO2- were relative to the substrate L-arginine used. NO2- and citrulline formation in the 2nd did not vary much from first run. The fil- trates were used for determination of iron leaching. The thio- late ligation of SR during the oxidation reaction was confirmed by measuring the EPR spectrum before and after the reaction at 2788. There was no change in the FT–IR spectra and XRD pattern of the recovered catalyst (data not shown).
TABLE 1
Stoichiometry of citrulline to NO2− formation from L-arginine using heme-thiolate SR complex
Experiment heme-thiolate
![]()
[citrulline]b
[NO2−]d
[arginine] c
[citrulline]:
[2] M. Sono, M. P Roach, E. D Coulter, J. H. Dawson, “Heme-Containing Oxy-
![]()
![]()
a All values are the average of duplicate measurements.
b citrulline formation and c arginine consumption were determined by HPLC methodology as described in the experimental section
d [NO2−] was determined by quantitation of NO2− with Griess reagent as described in
the experimental section
e 0.275 mmol of heme-thiolate complex was loaded
f 0.412 mmol of heme-thiolate was loaded g 0.550 mmol of heme-thiolate was loaded h 0.688 mmol of heme-thiolate was loaded
CONCLUSION
In summary a stable model for nitric oxide synthase activity using shunt mechanism has been developed, which showed the formation of nitrite and citrulline. The model consists of water-soluble heme-thiolate SR complex. This system is stable under the treatment of peroxide devoid of the degradation of the porphyrin moiety (active site). SR complex can be stored at room temperature under air for several months. L-arginine, instead of Nδ-hydroxy-L-arginine (L-NHA) was taken as the substrate for oxidation. It is highly probable that thiolate axial ligation alters the reactivity of the active intermediate of heme so as to make L-arginine oxidation much more favorable than chloride or imidazole axial ligation. The acceleration of the catalytic reaction by thiolate ligation is undoubtedly due to the enhancement of O-O bond scission because the concentration of peroxides is high so O-O bond cleavage step is the rate de- termining. The cyclic voltammogram of SR complex in DMF showed much more negative reversible reduction couple than iron porphyrin coordinated to chloride. The negativity of re- dox potential of SR is probably due electron donation between thiolate to the iron atom. Further investigation to characterize the structure of active intermediate of heme–thiolate complex spectroscopically is in progress in our laboratory.
ACKNOWLEDGMENTS
The author wish to sincerely thank
Department of Science & Technology (Project No:
SR/FT/CS-006/2009), Govt. of India for financial as-
sistance.