International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 407
ISSN 2229-5518
Kinetics of Batch Adsorption of Iron (II) ions from
Aqueous Solution using Activated carbon from
Strychnos Nux-Vomica L
S Arivoli1*, V Marimuthu1 and A R Mohamed Jahangir1
1* Department of Chemistry, Thiru.Vi.Ka. Government Arts College Thiruvarur,Tamil Nadu, India.
*Authors for Correspondence:arivu3636@yahoo.com
Abstract-Strychnos Nux-Vomica L leaves obtained from nearby pudukkottai district, having the particle size (53–150 μm) was used as an adsorbent for the removal of Fe (II) ion from aqueous solution. The effect of various factors (temperature, adsorbent dose and Initial pH) on adsorption of Fe (II) on Strychnos Nux-Vomica L was investigated. The effect of pH shows that the amount adsorbed increased with the increase of pH of solution. The equilibrium adsorption isotherms were analyzed by Langmuir and Freundlich equations. Both Langmuir and Freundlich models can describe the adsorption equilibrium but the Langmuir model shows better agreement. The amount adsorbed increased with the increase of temperature suggests the formation of dimer in the contact region. SEM micrographs and differential molar isosteric heat of adsorption (ΔH) calculated at different surface coverage, indicate that the surface is heterogeneous having energetically different adsorption sites. Values of n calculated from Freundlich plots indicate that adsorption of Fe (II) on Strychnos Nux-Vomica L is spontaneous. At high surface coverage, the differential heat of adsorption versus surface coverage plot shows maximum value indicating the occurrence of structural rearrangement in the adsorbate. With the increase of adsorbent dose, amount adsorbed increased due to the increased surface area of adsorbent.
Keywords: Iron, Activated Strychnos Nux-Vomica L Nano Carbon (ASNVL), Adsorption models, Equilibrium.
IJSEwastewater [10] tRhat is due to that activated carbon has a pore size
1. INTRODUCTION:


Water is the main component for living organisms, and the increase in water pollution as a result of progress in the industrial technologies, has been reduced using many methods to treat the wastewater [1]. The choice of suitable methods is controlled by different factors such as the efficiency of removing the pollutant materials, the availability of the used chemicals and the chemistry of the contaminated materials beside the process cost [2]. It is well– known that the pollutants in wastewater discharge from industrial effluents, sewage, sludge, pesticides, and fertilizers. The composition of the contaminated water depends on the source of the pollutant, the chemical composition of the original water whether it is surface or underground water and then the chemical reaction with the soil. For example, groundwater contains one or more contaminants like iron, manganese, ammonium, methane and natural organic matter, e.g., humic acid. Hence, before using this water supply for agro-irrigation purposes, these contaminants should be removed or reduced to the acceptable levels. Iron and manganese, which are usually present in the groundwater as divalent cations, considered to be contaminants mainly due to their organoleptic properties. The maximum recommended levels of Fe in drinking water are 0.3 mg/L, respectively. There are various methods for removing Fe (II) cations from the wastewater including ion-exchange method [3], oxidation by oxidizing agents such as chlorine and potassium permanganate [4], activated carbon and/or other filtering materials [5-7], supercritical fluid extraction (Andersen and Bruno, 2003), bioremediation [8], and treatment with limestone [9]. Some of these methods are simple and economic while the others are complicated and expensive. In oxidation treatment, oxygen, chlorine or potassium permanganate (KMnO4), is generally used for Fe (II) oxidation. Adsorption using activated carbon is an effective technique to remove heavy metals from
distribution which control its adsorption capacity, a chemical structure that influences its interaction with polar and non-polar adsorbate, and active sites which determine the type of chemical reactions with other molecules [11].
However, in developing countries such as India, traditional activated carbon remains an expensive material for heavy metal removal. Recently, many researchers have been published in the literature including preparation of activated carbons from various cheaper and alternative materials, e.g., agricultural by-products and biomass materials, using chemical activation with H3 PO4 [12-14]. However, there is only limited research on the preparation of activated carbons from woody biomass such as sawdust for uptaking heavy metals such as Hg (II) from wastewaters [15, 16] reported that the removal of Fe, Mn and Cu ions from acid mine drainage (AMD) by precipitation with NaOH depends on the pH value besides the oxidation state of the removed cations. On the other hand, various authors found that the removal of iron and other heavy metals by activated carbon depends on the nature of carbon (porosity, surface area, oxygen functional groups, etc.). Within the frame of this policy, the present paper narrates the investigation of Fe (II) ions removal from aqueous solution using adsorption methods in order to determine the optimum pH for the effective removal. The adsorption was carried out using an activated carbon obtained from Strychnos Nux-Vomica L leaves. The adsorption data was analyzed by using Langmuir and Freundlich isotherm models. Overall, this study was also intended to determine the efficiency and the optimum conditions in adsorption processes for removal of iron cations.
2. MATERIALS AND METHODS:
2.1. Adsorbent
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 408
ISSN 2229-5518
The natural plant material Strychnos Nux-Vomica L, used in the present investigations was collected from Edayappatti nearby pudukkottai the leaves were washed with distilled water several times to remove the dirt and dust and was subsequently dried in a hot air oven at 1100C. Afterward, carbonization of the Strychnos Nux-Vomica L was carried out at 6500C for 1 hour in a muffle furnace. A linsang nitrogen (purity 99.99%) flow of 150ml/min was maintained throughout the process of carbonization primary carbon was obtained on carbonization, which was afterward mixed with Zinchloride. Zinc chloride acts as a catalyst in the process. The primary carbon was activated at 1200oC for 6 hrs under optimized conditions to obtain activated nano carbon. The activated carbon was thereafter looked to room temperature in an insert atmosphere of nitrogen and washed with hot distilled water and 0.5 N Hydrochloric and until the pH of the material reached 7.0 the activated carbon was also dried in a hot air oven at 1100C, ground and sieved to obtain the desired particular size (150µm) and stored in desiccators for further use.
2.2. Chemicals

All chemicals used of high purity commercially available Analar grade purchased from scientific equipment company trichy. Iron solution was prepared from FeSO4 .7 H2O (2.489 g in 500 ml distilled water equivalent to one gram/liter). All experimental solutions were prepared by diluting the stock solution to the required concentration. The pH of each experimental solution was
The effect of pH on the rate of adsorption was investigated using iron concentration of 25 mg/L constant ASNVL dosage. The pH values were adjusted with dilute HCl and NaOH solution. The adsorbent – adsorbate mixture was shaken at room temperature using agitation speed (200 rpm) for 60 minutes. Then the concentration of Iron in solution was determined.
3. RESULT AND DISCUSSION
3.1 Characterization.
The different chemical constituents of activated Strychnos nux- vomica L are given in Table 1 along with some other characteristics. X-ray spectra of both adsorbents do not show any peak indicating the amorphous nature of activated Strychnos nux-vomica L.
adjusted to the required initial pH value using dilute HCl (or) NaOH before mixing the adsorbent. The concentration of residual Iron (II) was determined with atomic absorption spectrophotometer (Perkin
Elemer 2380).
2.3. Batch experiments
The effect of various parameters on the removal of Iron (II) onto ASNVL was studied batch adsorption experiments were conducted at (30-60°C). For each experimental run, 50 ml of iron solution of known initial concentration and pH were taken in a 250 ml plugged conical flask. A 25 mg adsorbent dose is added to the solution and mixture was shaken at constant agitation speed (200 rpm) sample were withdrawn at appropriate time intervals (10-60 min) and the adsorbent was separated by filtration. The residual solutions were analyzed to determine the Iron (II) concentration.
The effect of dosage of adsorbent on the removal of Iron (II) was measured by contacting 50 ml of 50 mg/L of Iron (II) solution with 25 mg of ASNVL till equilibrium was attained.
Adsorption equilibrium isotherm is studied using 25 mg of ASNVL dosage per 50 ml of Iron (II) solution. The initial concentration were ranged from (25 to 125 mg/L) in all sets of experiments. The plugged conical flask was shaken at a speed of
200 rpm for 60 minutes. Then the solution was separated from the mixture and analyzed for Iron (II) concentration. The adsorption capacity was calculated by using a mass equilibrium equation as follows:
qe = (C0 - Ce ) V/M ………………………. (1)
Where C0 and Ce being the initial iron concentration (mg/L) and equilibrium concentration, respectively V is the experimental volume of Iron (II) solution expressed in liters [L] and M is the adsorbent mass expressed in grams [g]. The Iron (II) ions percentage can be calculated as follows:
%R = (C0 – Ct ) x 100/C0 …………………. (2)
Table 1-Characteristics of the Adsorbent
Properties | ASNVL |
Particle size(mm) | 0.015 |
Density (g/cc) | 0.2005 |
Moisture content (%) | 0.2527 |
Loss in ignition (%) | 0.021 |
pH of aqueous solution | 5.2 |
3.2 Adsorption studies
Batch experiments were performed to investigate the adsorption process of Fe (II) by the ASNVL. For each experimental run, 50 mL of Fe (II) solution of known concentration, initial pH, ionic strength and the amount of the ASNVL were taken in a 250-mL stoppered conical flask. This mixture was agitated in a temperature-controlled shaking water bath at a constant speed of 200 rpm/min and certain temperatures. For adsorption equilibrium studies, Fe (II) solutions of different concentrations were contacted with a certain amount of ASNVL under certain conditions for an hr insuring the equilibrium was achieved. The residual Fe (II) concentration was then measured and the amount of Fe (II) adsorbed onto ASNVL was calculated from mass balance. Effects of contact time, adsorbent dosage, initial Fe (II) concentration, initial solution pH, ionic strength and temperature) on Fe (II) adsorption by ASNVL were investigated. Adsorption kinetics was determined by analyzing adsorptive uptake of Fe (II) from aqueous solution at different time intervals [18]. The amount of Fe (II) adsorbed at time t, qt (mg/g), was calculated using mass balance equation.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 409
ISSN 2229-5518
3.3 Effect of Contact Time on batch Adsorption of Iron (II)
ions in Aqueous Solution
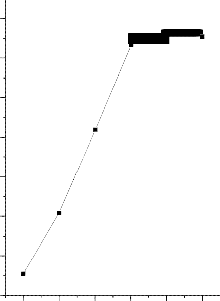
Figure 1 shows the effect of contact time on the adsorption of Iron (II) ions solution using activated nano carbon from Strychnos Nux-Vomica L. The concentrations of iron (II) ions in solution were varied from 25mg/L to 125mg/L and batch adsorption was carried out with 25mg of activated Strychnos Nux-Vomica L. The percentage of iron (II) ions adsorbed increased with time until equilibrium was reached for each concentrations. It is therefore evident from Fig 1 that at low concentration ranges the percent adsorption is high because of the availability of more reactive sites. At higher concentration of metal ion more and more surface sites are covered, the capacity of the adsorbent get exhausted due to non- availability of active surface sites. This leads to a fall in the percentage of metal ion adsorbed at higher concentration [19]. It was observed that the percentage adsorption of iron (II) ion rapidly reached equilibrium at 30 minutes of contact for 25mg/L
concentration, it increased to 100% implied that iron (II) (Fe2+) ion
was completely removed from aqueous solution at this concentration.
3.4 Effect of Initial Concentration on the Adsorption of Iron
(II) Ion in Aqueous Solution
The effect of initial concentration of iron (II) ions on adsorption
Effect of particle size of activated carbon produced from waste Strychnos Nux-Vomica L on the batch adsorption of iron (II) ions in aqueous solution. The adsorption of iron (II) ions increased with reduction in particle size [19]. The shape reduction also revealed that particle size of activated Strychnos Nux-Vomica L carbon has significant effect on the adsorption of iron (II) ions in aqueous solution for batch process. Smaller particle size (≤ 150 μm) adsorbed the highest amount of iron (II) ions within 30 mins of adsorption, hence for effective adsorption of iron (II) ions in aqueous solution using Strychnos Nux-Vomica L.
3.7 Effect of pH on the Batch Adsorption of Iron (II) Ions in
Aqueous Solution
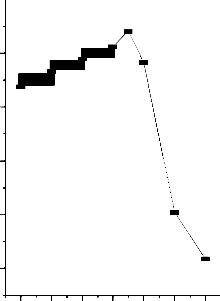
Effect of pH on Adsorption of heavy metals: The pH of the wastewater is one of the imperative factors governing the adsorption of the metal ions. The effect of pH was studied from a range of 2 to
6 under the precise conditions (at optimum contact time of 60 min,
200 rpm shaking speed, with 25mg of the adsorbents used, and at a room temperature of 30 oC). From Figure-3, with activated carbon from Strychnos Nux-Vomica L used as adsorbent, it was observed that with increase in the pH (2-6.5) of the wastewater, the percentage removal of iron ((II)) ions increased up to the pH 6.5 as shown above. At pH 6.5, maximum removal was obtained for metal ions, with 92.72% removal of Fe (II). The increase in percentage
removal of the metal ions may be explained by the fact that at
IJSER
of iron (II) ions using Strychnos Nux-Vomica L activated nano
carbon. Adsorption of irons (II) ions in solution increase significantly with reduction in the initial concentration of iron (II) ions in solution. The initial concentration of adsorbate varied from
25mg/L to 125mg/L. The rate of adsorption decreased from 92% -
70% as the concentration of iron (II) ions increased from 25mg/L to
125mg/L within 30 min of adsorption. This was expected and shows that there more reactive sites on the pore of Strychnos Nux-Vomica L activated carbon.
3.5 Effect of Carbon Dosage on the Batch Adsorption of Iron
(II) Ions in Aqueous Solution
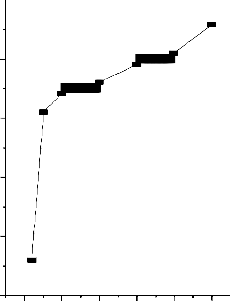
Iron (II) ions in aqueous solution of known concentration was adsorbed using different carbon dosage of activated Strychnos Nux- Vomica L ranging from 25mg – 125mg in 50 ml of stock solution of iron (II) ions . The effect of carbon dosage on the adsorption of iron (II) ions using activated carbon from waste Strychnos Nux-Vomica L leaves is presented in Fig 2. There was significant increase in the adsorption of iron (II) ions in solution as carbon dosage increased within adsorption time of 30min [14] reported similar findings during the removal of heavy metal adsorption by modified oak sawdust. This is due to the increased availability of active adsorption sites arising due to the increase in effective surface area resulting from the increases in dose of adsorbent or due to conglomeration of the adsorbent. Increasing the adsorbent dosage further, it was found that the optimum uptake of iron (II) ions requires about 250 mg of activated carbon from Strychnos Nux- Vomica L to adsorb 100% iron (II) ions in aqueous stock iron (II) solution.
3.6 Effect of Particle Size on the Batch Adsorption of Iron
(II) ions in Aqueous Solution
higher pH the adsorbent surface is deprotonated and negatively charge; hence attraction between the positively metal cations occurred [20].
3.8 Adsorption Models:
The adsorption equilibrium data were further analyzed into two well known isotherm models via Freundlich and Langmuir models.
3.9.1 Freundlich model:
The Freundlich model which is an indicative of surface heterogeneity of the adsorbent is described by the following equation.
Log qe = log kf + 1/n + log Ce (9)
Where Kf and 1/n are Freundlich constants associated with adsorption capacity and adsorption intensity respectively, The Freundlich plots between logqe and logCe for the adsorption of Fe (II) were drawn. It was found that correlation efficient values were less than 0.99 at both the temperature studied indicating that Freundlich model was not applicable to the present study.
3.8.2 Langmuir model:
The adsorption isotherm was also fitted to Langmuir model. The Langmuir equation which is valid for monolayer adsorption on to a surface is given below.
1/qe = 1/qm + 1/qmb Ce (10)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 410
ISSN 2229-5518
Where qe (mgg-1) is the amount adsorbed at the equilibrium concentration Ce (mol L-1), qm (mgg-1) is the Langmuir constant representing the maximum monolayer adsorption capacity and b (L mol-1) is the Langmuir constant related to energy of adsorption. The plots 1/qe as a function of 1/Ce for the adsorption of Fe (II) was found linear. Suggesting the applicability if Langmuir model in the present adsorption system. The correction coefficient (R2 = 0.9926
and 0.9932 at 30 & 60oC respectively for Langmuir model) confirm
good agreement between both theoretical models and our experimental results the values of the monolayer capacity (qm ) and equilibrium constant (b) have been evaluated from the intercept and slope of these plots and given in Table 3. It is adsorbent for the Fe (II) is comparable to the maximum adsorption obtained from the adsorption isotherms. These facts suggest that Fe (II) is adsorbed in the form of monolayer coverage on the surface of the prepared adsorbent [21]. Satisfactory fitting of the Langmuir model to the adsorption of Fe (II) on Activated Strychnos nux-vomica L adsorbent.
3.10 Kinetics study
The Kinetic adsorption data were evaluated to understand the dynamics of the adsorption reaction in terms of the order of the rate constant batch experiments were conducted to explore the rate of Fe (II) adsorption by Strychnos Nux-Vomica L as described in
adsorption isotherms section at pH 6.5. Three Kinetic models were
quite good for where the calculated correlation coefficients (R2)
almost the same values.
3.11 The Elovich equation and intra-particle diffusion model
The Elovich model equation is generally expressed as dqt / dt = α exp (-βq t ) ……..(10)
Where; α is the initial adsorption rate (mg g-1 min-1) and β is the desorption constant (g/mg) during any one experiment. To simplify the Elovich equation. [24] assumed αβt>>t and by applying boundary conditions qt = 0 at t= 0 and qt = qt at t = t Eq.(10) becomes:
qt = 1/β ln (αβ) + 1/β ln t …………… (11)
If Fe (II) ions adsorption fits with the Elovich model, a plot of qt
vs. ln(t) should yield a linear relationship with a slope of (1/β)and
an intercept of (1/β)ln (αβ). The Elovich model parameters α, β, and correlation coefficient (γ) are summarized in table 6. The experimental data such as the initial adsorption rate (α) adsorption constant (β) and the correlation co-efficient (γ) calculated from this model indicates that the initial adsorption (α) increases with temperature similar to that of initial adsorption rate (h) in pseudo- second–order kinetics models[22]. This may be due to increase the pore or active site on the ASNVL adsorbent.
For adsorption of Fe (II) on to Strychnos nux-vomica L the obtained results represent more conformity to pseudo-second order model (R2 =0.95), the initial adsorption rate K2 qe 2 for Strychnos
Nux-Vomica L. Kinetic data for the adsorption of Fe (II) were also
IJSER
applied to the adsorption Kinetic data in order to investigate the
behavior of adsorption process of Fe (II) onto the adsorbents. These
analyzed according to intra-particle diffusion model achieve can be
formulated as[25].
models include the pseudo first order Kinetics (reversible or
irreversible), the pseudo – Second – order and the intra particle diffusion models the linear form of reversible pseudo – first – order model can be formulated as:
Qt = k pt 0.5
(12)
ln ( qe -qt ) = lnqe - k x t
(7)
Where qt is the amount of Fe (II) adsorbed (mg/g) at time
t, and kp (mg/g min0.5) is the rate constant for intra – particle
diffusion. Results are shown in table 6. Usually the plot of qt versus
Where qe (mol/g) and qt (mole/g) are the amount of Fe (II)
adsorbed at equilibrium and at time t, respectively and K1 (min-1) is
the rate constant K1 values were evaluated from the linear
regression of ln(qe -q t ) versus data. Linear from of irreversible
pseudo first order model can be formulated as:
t0.5 may be distinguished in two or more steps taking place during adsorption process including instantaneous adsorption stage by external mass transfer (first sharper portion), intra-particle diffusion which is the rate controlling stage (second portion as the gradual
adsorption stage) and the final equilibration of age where the intra –
ln (Co / Ct ) = K × t
(8)
particle diffusion starts to slow down due to extremely low solute concentration in solution (the third portion).
3.12 Adsorption Thermodynamics
The thermodynamic parameters for the adsorption of Fe (II) ions
Where Co (mg/l) is the initial concentration of Fe (II) and Ct (mg/l)
is equilibrium concentration of Fe (II) at time‘t’ respectively, and
Kg min-1 is the rate constant Evaluation of data has been done using linear plot of ln (Co /Ct ) versus time. The Linear form of pseudo – Second – order equation can be formulated us.
by Activated Strychnos nux-vomica L were determined using the following equations:
D
t/ q = 1 / K q
2 + t / q
(9)
Where KD is the distribution coefficient for the adsorption in
t 2 e e
Where qe and qt are surface loading of Fe (II) at equilibrium and time ‘t’ respectively and K2 (g/mg/min) is the second – order rate constant, The Linear plot of t/qt as a function of provided not only the rate constant K2, but also an independent evaluation of qe . The fitting of experiment al data to the pseudo – first – order and the pseudo – second- order equation seemed to be
g/L, ΔGo is the Gibbs free energy in J/mol,
R is the universal gas constant in J/mol K, T is the absolute temperature in K, ΔSo is the entropy change in J/mol K and ΔHo is the enthalpy change in kJ/mol [23]. The values of Gibbs free energy (ΔGo) for various temperatures were calculated from the experimental data. The values of enthalpy change (ΔHo) and entropy change (ΔSo) were estimated from the slope and intercept of the plot of ln KD Vs 1/T. The estimated thermodynamic parameters were tabulated and shown in table 5. But the negative values of Gibbs
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 411
ISSN 2229-5518
free energy change (ΔGo) obtained for the adsorption of Fe (II) ions by Activated Strychnos nux-vomica L at various temperatures had shown the spontaneous nature of the adsorption process.
The positive values of enthalpy change (ΔH°) obtained for the adsorption of Fe (II) ions by Activated Strychnos nux-vomica L at various temperatures indicated that the adsorption reactions were endothermic. The positive values of entropy change (ΔS°) for the adsorption of Fe (II) ions by Activated Strychnos nux-vomica L at various temperatures showed the increased randomness at solid liquid interphase during the sorption processes of Fe (II) ions on the adsorbent ASNVL. This is a direct consequence of (i) opening up of structure of adsorbent beads (ii) enhancing the mobility and extent of penetration within the adsorbent beads and (iii) overcoming the activation energy barrier and enhancing the rate of intra-particle diffusion [25].
The adsorption of Fe (II) ions by Activated Strychnos nux- vomica L slightly increased when temperature was raised up to 60 oC. It might be due to the generation of new active sites on the adsorbent surface and also due to the increased rate of pore diffusion. But when the temperature was further raised, adsorption processes had decreased largely. It showed that the adsorption processes of Fe (II) ions by Activated Strychnos nux-vomica L were exothermic reactions and physical in nature which involved the weak forces of attraction between the sorbate-sorbent molecules.
3.13. SEM Images of APSNC
pseudo second order kinetic equations, and intra-particle diffusion models. Sorption kinetics showed good agreement of the experimental data the pseudo second order kinetic reaction is the rate controlling step with some intra particle diffusion taking place.
The high adsorption intensity of Strychnos Nux-Vomica L activated carbon and its affinity for Iron (II) ions can help solve many adsorption challenges in the industry and in water purification processes.
ACKNOWLEDGEMENT
The authors sincerely thank the University Grants Commission
New Delhi for providing the fund from Major Research Project.
REFERENCE:
[1] Chapman D., 1992. Water quality assessments: A guide to the use of biota, sediments and water in environmental monitoring. Kluwer Academic Publishers, Dortrecht, pp:
372.
[2] Letterman, R.D., 1999. American Water Works Association: Water Quality and Treatment. 5th ed., McGraw- Hill, New York.
[3] Vaaramaa, K. and J. Lehto, 2003. Removal of metals and anions from drinking water by ion exchange. Desalination., 155: 157-170.
[4] Ellis, D., C. Bouchard and G. Lantagne, 2000. Removal of
IJSER
The SEM images of ASNVL (Fig.5a and 5b) Shows the SEM
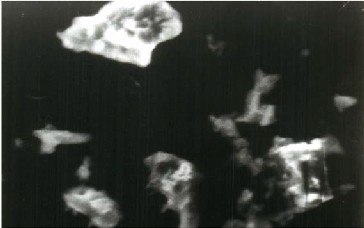
micrographs of ASNVL sample before and after dye adsorption. It is clear that ASNVL has considerable numbers of heterogeneous layer of pores where there is a good possibility for Fe (II) ions to be adsorbed. The surface of metal-loaded adsorbent, however, clearly shows that the surface of ASNVL is covered with metal ions.
4. Desorption studies:
Desorption studies help to elucidate the nature of adsorption and recycling of the spent adsorbent and the metal ions. If the adsorbed metal ions can be desorbed using neutral pH water, then the attachment of the metal ion of the adsorbent is by weak bonds. The effect of various reagents used for desorption studies. The results indicate that hydrochloric acid is a better reagent for desorption, because we could get more than 92% removal of adsorbed metal ion. The reversibility of adsorbed metal ion in mineral acid or base is in agreement with the pH dependent results obtained. The desorption of metal ion by mineral acids and alkaline medium indicates that the metal ion was adsorbed onto the ASNVL through
physisorption as well as by chemisorptions mechanisms20.
CONCLUSION
Kinetics of batch adsorption of iron (II) ions from aqueous solution using activated carbon from waste Strychnos Nux-Vomica L has been investigated. The amount of iron (II) ions adsorbed was found to vary significantly with process parameters such as particle size, carbon dosage, initial concentration of adsorbate and contact time. The adsorption process follows Langmuir and Freundlich isotherms but a better sorption fit using Langmuir isotherm model was obtained indicating a monolayer formation over a surface of the material. The monolayer saturation capacity of 166.7 mg of iron (II) ions adsorbed per g of Strychnos Nux-Vomica L activated carbon was obtained and found to be higher than monolayer saturation capacity of other adsorbent used for iron (II) ions adsorption. Adsorption kinetics was modelled using the pseudo first order,
IJSER © 2013
iron and manganese from groundwater by oxidation and
microfiltration. Desalination., 130: 255-264.
[5] bin Jusoh, A., W.H. Cheng, W.M. Low, A. Nora’aini, and M.J.M.M. Noor, 2005. Study on the removal of iron and manganese in groundwater by granular activated carbon. Desalination., 182: 347-353.
[6] Munter, R., H. Ojaste, and J. Sutt, 2005. Complexed iron removal from groundwater. Journal of Environ. Eng.,131:
1014-1020.
[7] Okoniewska, E., J. Lach, M. Kacprzak and E. Neczaj,
2007. The removal of manganese, iron and ammonium nitrogen on impregnated activated carbon. Desalination.,
206: 251-258.
[8] Berbenni, P., A. Pollice, R. Canziani, L. Stabile, and F.
Nobili, 2000. Removal of iron and manganese from hydrocarbon-contaminated groundwaters. Bioresour. Technol., 74: 109-114.
[9] Aziz, H.A., M.S. Yusoff, M.N. Adlan, N.H. Adnan, and S.
Alias, 2004. Physico-chemical removal of iron from semiaerobic landfill leachate by limestone filter. Water Manag., 24: 353-358.
[10] Ahmedna, M., W.E. Marshall, A.A. Husseiny, R.M. Rao, and I. Goktepe, 2004. The use of nutshell carbons in drinking water filters for removal of trace metals. Water Res., 38: 1062-1068.
[11] El-Sherif, I. Y. and N.A. Fathy, 2011. Equilibrium and kinetic study of Cd (II) and Ni (II) ions removal by semi– carbonized/H3PO4 cotton stalks. EJEAFChe., 10: 2744-
2758.
[12] Girgis, B.S., A.A. Attia, and N.A. Fathy, 2007.
Modification in adsorption characteristics of activated carbon produced by H3PO4 under flowing gases. Colloids and Surfaces A: Physicochem. Eng. Aspects., 299: 79-87.
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 412
ISSN 2229-5518
[13] Girgis, B.S., A.M. Soliman, and N.A. Fathy, 2011.
Development of micro-mesoporous carbons from several seed hulls under varying conditions of activation. Microporous and Mesoporous Materials., 142: 518-525
[14] Kazemipour, M., M. Ansari, S. Tajrobehkar, M.
Majdzadeh, and H.R. Kermani, 2008. Removal of lead, cadmium, zinc, and copper from industrial wastewater by carbon developed from walnut, hazelnut, almond, pistachio shell, and apricot stone. Journal of Hazardous Materials, 150: 322-327.
[15] Budinova, T., E. Ekinci, F. Yardim, A. Grimm, E.
Björnbom, V. Minkova, and M. Goranova, Characterization and application of activated carbon produced by H3PO4 and water vapor activation. Fuel Processing Technology, 87: 899-905.
[16] Balintova, M. and A. Petrilakova, 2010. Study of pH influence on selective precipitation of heavy metals from acid mine drainage. In 14th Conference on Environmental and Mineral Processing, VSB-TU, Ostrava,Czech, Republic, pp: 160-166.
[17] Hamadi N K, Swaminathan S, Chen X D, 2004.
Adsorption of paraquat dichloride from aqueous solution by activated carbon derived from used tires. Journal of Hazardous Materials, 112(1-2): 133–141.
[18] Shrivastava, R.K., Ayachi, A.K. and Mora, M. 2001.
[19] Namasivayam, C. and Holl, W.H. 2004. Chromium (III) removal in tannery wastewaters using Chinese reed (Miscanthus Sinensis), a fast growing plant. Holz. Roh. Werkst. 62: 74 – 80.
[20] Sharma, A. and Bhattacharya, D. 2005. Utilisation of biosorbent based on Azadirachta indica (Neem) leaves for removal of water soluble Metals. Indian J Environ. Chem.
12: 285-295.
[21] Singh, I.B. and Singh, D.R. 2001. Hexavalent chromium removal using iron bearing industrial sludges: Indian J. Chem. Technol. 8: 487-495.
[22] Jambulingam, M., Rehugadevi, N., Karthikeyan, S., Kiruthika, J., Patabhi. S. 2005. Adsorption of Cr(VI) from aqueous solution using a low cost activated carbon. Indian J Environ. Protect. 25(5): 458-63.
[23] Ngah W.S.W, Endud C.S, Mayanar R. (2002) Removal of copper(II) ions from aqueous solution onto chitosan and cross-linked chitosan beads React. Funct. Polym. 50, 181-
190
[24] Chien S H, Clayton W R, “Application of Elovich Equation to the kinetics of Phosphate release and sorption on soil”, Soil Sci. Sco, Am. J. 44 :265 – 268, 1980
[25] Weber W J, Morris J C, “Kinetics of adsorption on Carbon from solution”. J, Sanitary Eng, Div. 90, 79, 1964.
Removal of Cr (VI) by utilization of Bidi leaves. Pollut. Res. 20(4): 639 – 643).
TABLE: 2. EQUILIBRIUM PARAMETERS FOR THE ADSORPTION OF IRON (II) ION ONTO ASNVL
M0 | Ce (Mg / L) | Qe (Mg / L) | Removal % |
M0 | 30oC | 40oC | 50oC | 60oC | 30oC | 40oC | 50oC | 60oC | 30oC | 40oC | 50oC | 60oC |
25 | 3.64 | 3.03 | 2.54 | 2.73 | 92.70 | 93.92 | 94.90 | 94.52 | 92.72 | 93.92 | 94.90 | 94.52 |
50 | 11.43 | 10.46 | 8.47 | 7.56 | 177.1 | 179.0 | 183.0 | 184.8 | 88.56 | 89.53 | 91.52 | 92.43 |
75 | 25.74 | 22.36 | 19.64 | 17.36 | 248.5 | 255.2 | 260.7 | 265.2 | 82.83 | 85.08 | 86.90 | 88.42 |
100 | 47.84 | 43.64 | 19.64 | 36.67 | 304.3 | 312.7 | 360.7 | 326.6 | 76.07 | 78.17 | 90.17 | 81.66 |
125 | 73.88 | 69.93 | 40.64 | 60.90 | 352.2 | 360.1 | 418.7 | 378.19 | 70.44 | 72.02 | 83.74 | 75.63 |
TABLE: 3. LANGMUIR AND FREUNDLICH ISOTHERM PARAMETER FOR THE ADSORPTION OF IRON (II) ION ONTO ASNVL
Temp. (oC) | Langmuir Parameters | Freundlich Parameters |
Temp. (oC) | Qm | b | Kf | n |
30oC | 414.34 | 0.0662 | 1.748 | 2.2700 |
40oC | 419.07 | 0.0766 | 1.791 | 2.3217 |
50oC | 565.63 | 0.0634 | 1.758 | 1.8187 |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 413
ISSN 2229-5518
60oC | 437.88 | 0.0931 | 1.833 | 2.2802 |
TABLE: 4. DIMENSIONLESS SEPERATION FACTOR (RL ) FOR THE ADSORPTION OF IRON (II) ION ONTO ASNVL
(Ci ) | Temperature °C |
(Ci ) | 30oC | 40oC | 50oC | 60oC |
25 | 0.2318 | 0.2068 | 0.2395 | 0.1768 |
50 | 0.1311 | 0.1153 | 0.1360 | 0.0969 |
75 | 0.0914 | 0.0799 | 0.0950 | 0.0668 |
100 | 0.0701 | 0.0612 | 0.0730 | 0.0509 |
125 | 0.0569 | 0.0495 | 0.0592 | 0.0411 |
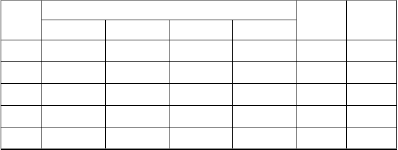
TABLE: 5. THERMODYNAMIC PARAMETER FOR THE ADSORPTION OF IRON (II) ION ONTO ASNVL
∆Go
(C0 ) | 30oC | 40oC | 50oC | 60oC | ∆H° ∆S° |
25 | -5786.1 | -6346.5 | -6702.7 | -6972.1 | 6.11 | 39.51 |
50 | -4319.1 | -4798.5 | -5878.6 | -6068.4 | 14.96 | 63.61 |
75 | -3485.4 | -3821.6 | -4392.9 | -4953.7 | 11.56 | 49.47 |
100 | -2250.9 | -2459.8 | -5293.1 | -3194.0 | 15.58 | 59.39 |
125 | -1289.8 | -1613.2 | -3613.3 | -2199.3 | 13.52 | 49.38 |
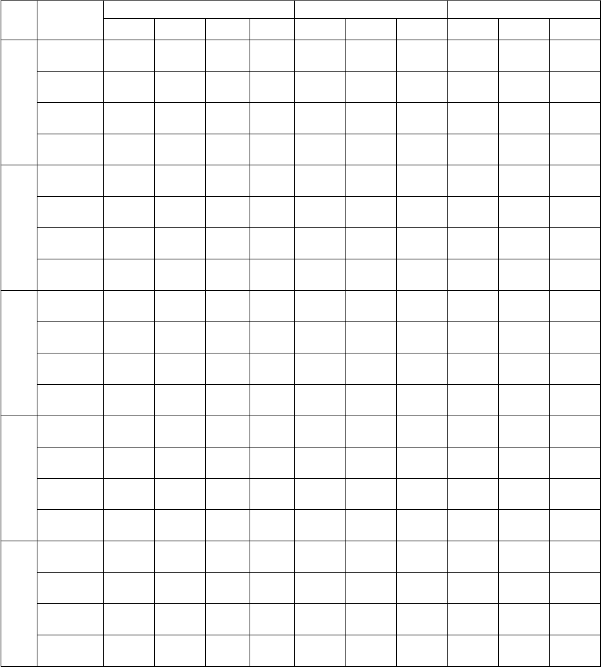
TABLE: 6. THE KINETIC PARAMETERS FOR THE ADSORPTION OF IRON (II) ION ONTO ASNVL
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 414
ISSN 2229-5518
Pseudo second order Elovich model Intraparticle diffusion
C0 Temp °C
e
K2 γ h α β γ Kid γ C
25
50
75
100
125
30 99.490 0.0018 0.994 19.52 818.20 0.0916 0.9959 1.7335 0.9981 0.1291
40 100.17 0.0016 0.991 20.90 1203.6 0.0951 0.9968 1.7504 0.9975 0.1225
50 100.73 0.0014 0.992 20.87 1901.9 0.1004 0.9982 1.7651 0.9969 0.1145
60 100.07 0.0014 0.991 22.10 2783.7 0.1052 0.9948 1.7738 0.9973 0.1092
30 190.41 0.0021 0.992 34.85 1260.1 0.0468 0.9961 1.7045 0.9989 0.1329
40 192.37 0.0020 0.991 35.97 1460.5 0.0471 0.9987 1.7145 0.9928 0.1302
50 195.86 0.0018 0.993 38.53 1968.7 0.0478 0.9967 1.7332 0.9941 0.1251
60 197.17 0.0017 0.991 40.57 2144.5 0.0477 0.9989 1.7400 0.9948 0.1242
30 266.65 0.0022 0.992 51.10 2109.2 0.0341 0.9959 1.6825 0.9952 0.1296
40 273.50 0.0022 0.994 52.60 2279.9 0.0334 0.9984 1.6953 0.9983 0.1286
50 280.24 0.0021 0.991 55.94 2501.0 0.0328 0.9967 1.7095 0.9960 0.1276
60 282.68 0.0020 0.992 47.44 4605.9 0.0362 0.9983 1.7245 0.9940 0.1143
30 329.80 0.0024 0.994 54.09 1150.9 0.0248 0.9943 1.6127 0.9946 0.1472
0.995 0.9982 0.9988
40 338.49 0.0024 56.28 1394.5 0.0248 1.6317 0.1429
50 343.90 0.0011 0.997 59.50 1673.6 0.0249 0.9972 1.6468 0.9954 0.1393
60 351.13 0.0023 0.999 66.09 2414.7 0.0254 0.9969 1.6716 0.9990 0.1324
30 385.32 0.0022 0.998 57.66 839.01 0.0199 0.9981 1.5570 0.9987 0.1600
40 392.62 0.0025 0.997 61.02 1034.8 0.0201 0.9948 1.5764 0.9967 0.1545
50 405.44 0.0016 0.998 58.86 843.37 0.0188 0.9994 1.5756 0.9961 0.1609
60 410.50 0.0025 0.992 65.92 1286.2 0.0197 0.9972 1.6052 0.9952 0.1499
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 415
ISSN 2229-5518
85
80
75
70
65
60
55
50
10 20 30 40 50 60
Contact Time in min
Fig:1-Effect of Contact Time on the Removal of Metal ion
[M]=50 mg/L; adsorbent dose=25mg/50ml; pH-6.5;Temp 300C
100
90
80
70
60
50
0 50 100 150 200 250
Adsorbent dose in mg
Fig.2-Effect of adsorbent dose on the removal of Metal Ion
[M]=50mg/L: Contact time=60 min: pH=6.5: Temp 300C
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 416
ISSN 2229-5518
90
80
70
60
50
40
3 4 5 6 7 8 9
Initial pH
Fig.3-Effect of Initial pH on the removal of Metal Ion
[M]=50mg/L: Contact time=60 min: dose=25mg/50ml
85
80
75
70
65
60
0 20 40 60 80 100
Con. of other ion in mg/L
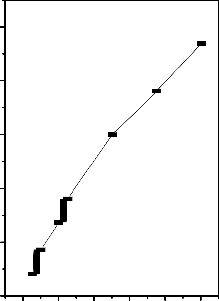
Fig.4-Effect ionic strength on the adsorption of Metal ion
[M]=50 mg/L;Contact time=60 min;Dose=25 mg/50 ml
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 417

ISSN 2229-5518
Fig.5.a- SEM image of ASNVL before adsorption Fig.4.b-SEM image of ASNVL after the adsorption of Fe (II) ions
IJSER
IJSER © 2013 http://www.ijser.org








