example, there are some effective factors on extraction
reaction speed such as sulfuric acid concentration,
International Journal of Scientific & Engineering Research Volume 2, Issue 8, August-2011 1
ISSN 2229-5518
Kinetics from Reaction of Copper Extraction from Convertor Dust in Sarcheshmeh Copper Complex, Kerman
Sajadeh Riahi Madvar, Esmaeil Eynalian
Abstract - In this study the kinetics of copper extraction from convertor dust in Sarcheshmeh Copper Complex in Kerman has been presented based upon the shrinking core. In this research we examined different parameters such as acid, temperature, pressur e, time, size of particles and stirring speed. The laboratory results show that stage of reaction control is the surface reaction on particles.
Index Terms – Acidity, Copper Complex, Extraction, Kinetics, Partial Pressure, Surface reaction, Thermodynamic parameters
—————————— ——————————
CuS Fe2
(SO4 )3
CuS CuSO4
2FeSO4
OR the optimal conditions of copper recovery by the designed pressurized reactor, it is necessary
CuS Fe2 (SO4 )3 S CuSO4 2FeSO4
4FeSO4 O2 2H 2 SO4 2Fe2 (SO4 )3 2H 2 O
to know the kinetics of extraction reaction. For
example, there are some effective factors on extraction
reaction speed such as sulfuric acid concentration,![]()
S 3 O
2 2
H 2
O H
2 SO4
reaction temperature, pressure, time, stirring speed
CuS Fe(SO4 )3 CuSO4 2FeSO4
and size of particles which their effects must be
2FeSO
1 O
H SO
Fe SO
H O
measured on isolation speed. The sample analysis showed that the dust compositions are similar to![]()
4 2 2 2 4
3
2 ( 4 )3
2
The
phosphoric ores and there have been a lot of works done on kinetics of reaction so far, for example, Achorjee (1) works on examining kinetics of lead sulfide extraction reaction with chloride acid and Pritker (2) works on kinetics of lead sulfide extraction reaction with chloride acid. Other researches included on the field are Nunez (5) work who studied in details on solving zinc ferric compositions in chloride acid and Lanyon (6) and his colleagues examined iron extraction from its oxide sources with chloride acid and also Olani Pekun (7) defined the effective factors in kinetics of extraction.
The mechanism of extraction reaction based upon the
shrinking model:
If we have the reaction with the following equations: The copper sulfide compositions reaction with![]()
S 2 O2 H 2O H 2SO4
copper oxide compositions reaction with sulfuric acid:![]()
CuCO3Cu(OH ) 2 2H 2 SO4
2CuSO4 CO2 3H 2 O
CuCl2 .3Cu(OH ) 2 3H 2 SO4
3CuSO4 CuCl2 6H 2 O CuSiO3 .2H 2 O H 2 SO4 CuSO4 SiO2 3H 2 O
CuSO4 .3Cu(OH )2 3H 2 SO4 4CuSO4 6H 2O
Cu2O H 2 SO4
Cu H O CuSO
2 4
sulfuric acid and oxygen:![]()
The direct reaction of sulfide compositions:
17 1![]()
![]()
CuFeS 2 4 O2 2 H 2 O4
CuS 2O2 CuSO4 1 1
![]()
![]()
CuSO4 2 Fe2 (SO4 ) 3 2 H 2 O
The indirect reaction of sulfide compositions:
IJSER © 2011
International Journal of Scientific & Engineering Research Volume 2, Issue 8, August-2011 2
ISSN 2229-5518
CuFeS 2 2Fe2 (SO4 ) 3
CuSO4 5FeSO 4 2S
5FeSO 4
![]()
5 O
4 2
H
2 SO4
![]()
5 Fe
2 2
(SO4 ) 3
![]()
5 H O
2 2
2S 3O2 2H 2 O2 2H 2 SO4
There have been many studies achieved on solid-fluid reaction which divided into two groups (8, 9, 10).
When the reaction in liquid phase produces a very soluble material, the following equation shows the reaction which takes on solid level:![]()
in which RA is the reaction speed for area unit representing a function of fluid elements. The initial and real diameter and particles represented by d0 and d, respectively. If d3/d03 ration shows as (1-X), then X is a solid part participating in the reaction. Thus N=V0 ρ (1-X)/M and we have:![]()
in which M shows the solid molecular weight and ρ is the solid density. Therefore we can see the reacted part is appropriate with the initial diameter of particles. By integration the above equation is written as the following formula:![]()
![]()
by which we can get the reacted part. If the X curve is perpendicular to time, then we can say that the surface reaction is controller [18,19, 20].
In this state assuming there is a balance on surface, speed in base of surface is appropriate with concentration difference on surface and penetration into the product and if B shows the penetrated material, the reaction speed which equals to penetration speed represents by the following formula:![]()
in which s shows the layer thickness of product and
A=π (d0 – 2s)2 shows the surface of particles.
Fig 12-3: Local view of changes in particles
Therefore the mass flux is written by the following formula: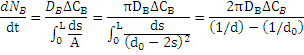
in which d is the diameter of particle and the particle value participated in the reaction is related to the particle value by the following formula:![]()
![]()
in which it also shows the product value is appropriate with the initial diameter of particle. If we integrated the formula then we have:![]()
If the curve of this function is a direct line by time thus the penetration from inside of product is controller. As we may know the speed chemical reactions is functional to different parameters of which the most important ones are pressure, temperature, concentration of materials, and if there is any solid particles, the size of particles. So the relationship of speed may be written as follow:![]()
![]()
in which r shows the speed of chemical reaction in terms of mole by solid surface unit, T is temperature, P shows pressure and N is the number of moles. The Arrhenius equation shows the function of temperature.
in which E shows the energy of reaction activity, R is the gas fixed value and T is temperature. Since in solid phase reactions there are three phases of external penetration, internal penetration and chemical reaction, thus there are three phases in this system too and if the surface reaction is controller, the speed equation may be written as this:
IJSER © 2011
International Journal of Scientific & Engineering Research Volume 2, Issue 8, August-2011 3
ISSN 2229-5518
r = K. f (C)
in which K is a fixed value dependent to temperature
and in this reactions due to tendency from acid ideal state, it is a better parameter for defining the speed of reaction so that we can write the above formula as this:
r = K.f (aH+)
With this value the following methods have been used for determining the speed and effective factors on reaction.
As we said earlier, in this article we try to examine some effective factors on reaction speed of copper compositions. It is required to do some standard tests. So we used 98% sulfuric acid. In this test using a mixer with 900 rpm (fixed) mounted in a Bach reactor and reactor temperature was controlled by oil pump and reactor pressure was controlled by oxygen capsule, the effective factors have been examined on chemical reactions. Fig (2) shows how to pour a specific weight of solid sample at the time base into a container with acid and while keeping the mixed at a fixed round and temperature and container pressure are changing during testing, we took samples from the container for determining the reacted amount of copper and iron and after filtering by Atomic Absorption Spectrophotometer and some lab methods, we determine the iron and copper concentrations in samples which the results are shown as chart later in the article.
1- The dust of blast furnaces at Sarcheshmeh Copper Complex in Kerman containing high amounts of oxide copper compositions and some sulfide copper compositions which we should take proper method to recover them.
2- The leaching tests under pressure results showed that the consumption of acid by dust is high at first then it lowers and reached to a fixed value.
3- With the lab data and results, we can introduce
130°C temperature, 8 bar oxygen pressure, mixer speed at 900 rpm, 3 hours of time, 0.1 gr/lit of pulp density and 200 gr/lit of acidity as the optimal conditions for pressurized dissolution of blast furnaces at work. In these optimal conditions, the recovery rate of copper is 88%.
4- Analyzing the solid products showed that the solid contains about 3%-5% of copper. These copper compositions are unrecoverable due to their unknown structure and composition.
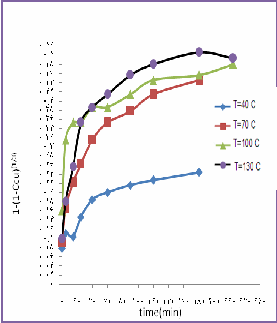
Chart (1): Determinant chart of reaction controller
This chart shows the surface reaction is controller. If
we draw the right side of equation (7) in terms of time
there would not any direct lines.
The effect of different parameters with the controlling surface reaction and using the tests can be summarized as follow:
IJSER © 2011
International Journal of Scientific & Engineering Research Volume 2, Issue 8, August-2011 4
ISSN 2229-5518
With the results in previous paragraphs, we can see
that the salvation under pressure is a proper method
for recovering copper from its sulfide compositions in dust, and if it has economic justifications, it can be a proper replacement for similar methods.
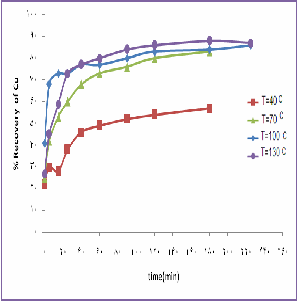
speed=900 rpm,Po2=8bar,S/L=0.1,acidite=200 g/l
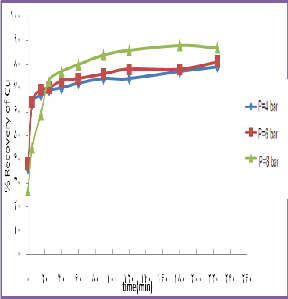
speed=900rpm,T=130 ˚C, Acidite =200 g/l, S/L=0.1
IJSER © 2011
International Journal of Scientific & Engineering Research Volume 2, Issue 8, August-2011 5
ISSN 2229-5518
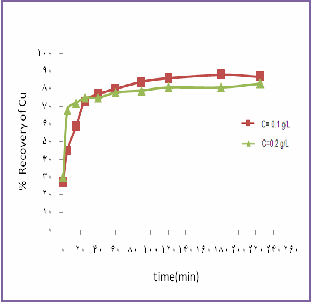
speed=900rpm,T=130˚C,Po2=8bar, Acidite =200g/l
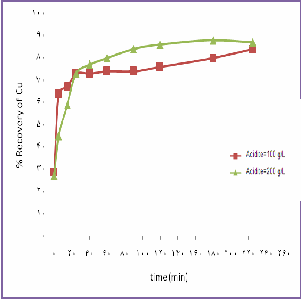
speed=900 rpm, T=130 C,Po2=8 bar ,S/L=0.1
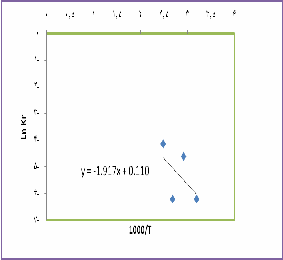
Q
![]()
From - R ratio we can calculate the activation energy equals to 15.94 kj/mol, which numerically shows the chemical control.
[1] Acharjee.D.K.(1989);"Recovery copper from copper concentrator tailing by leaching".Can.J.Chem.Eng.vol.67.pp.686-688
[2] Pritzker.M.(1998);"Model for the ferric chloride leaching of galena Metall.Trans B.,vol.29B,pp.953-
960
[3] Majima H.,Awakura Y.,Mishima T.,(1985);"the leaching of hematite in acid solutions":Metal.Trans.B,vol.26B,pp.23-30
[4] Majima H.(1995);"Importance of fundamental study in hydrometallurgy"; Metal.&Mat.Tras.B,vol. 26B,pp.1109-1122
[5] Nunez C.,Vinals J.(1984):"Kinetics of leaching of
zinc ferrite in aqueous hydrochloric acid solutions"; Metal.Trans.B,vol.15B
[6] Lanyon M.R.,Lwin T.,Merritt RR.,(1999);"The dissolution of iron inhydro choleric acid leach of an ilmenite concentrate"; Hydrometallurgy,vol.51(3),pp.299-323
[7] Olanipekun E.O.(2000);"Kinetics of leaching laterite"; Int.J.Miner.process.,vol.60.pp.9-14
[8] Smith J.,(1980);"Chemical Engineering
Kinetics".New York.Mc-Graw Hill,Inc.
[9] Fogler H.S.(1992) ; “Elements of Chemical Reaction Engineering”2nd editions , New Jersey , Perentice-Hall, Engelwood cliffs.Inc.
[10] Miller.G &Newton.T, “Copper Heap Leaching
Testing, Interpretation and Scale up”, ALTA 1999.
[11] Wiess N.L, “SME Mineral Processing Hand Book”
,1985
IJSER © 2011