International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 646
ISSN 2229-5518
Isolation, Purification and Characterization of Glucanase Enzyme from the Antagonistic Fungus Trichoderma
Biocontrol Lab, Department of Plant Pathology,
Chandra Shekhar Azad University of Agriculture and Technology, Kanpur, U.P. India
Corresponding Author: Sonika Pandey
Email: sonica.dey@gmail.com
Glucanase enzyme isolated from Trichoderma isolates were purified to homogeneity using ammonium persulphate precipitation and Fast Protein Liquid
Chromatography. Purity of the isolated enzyme was confirmed by SDS-PAGE. Enzymatic properties such as effect of temperature, pH, SDS and EDTA were determined. Molecular weight of Trichoderma isolates was found to be around 55 kDa. The optimum temperature for glucanase enzyme was 50˚C and the optimum pH was 5.0. SDS and EDTA were at a concentration 0f 20 µg/ml showing an inhibitory effect on glucanase enzyme activity.
—————————— ——————————
Trichoderma sp. are active mycoparasites against a
variety of soil borne pathogens. The antagonistic mechanism of Trichoderma is a complex process involving chemotropism [4], lectin-mediated recognition [11,12,13], and formation of trapping and penetration structures [6,7]. This process is further supported by the secretion of extracellular enzymes such as chitinases [2,5,10] β-glucanases, xylanase [10,17,18], and proteinases. These enzymes degrade the cell wall components of pathogens such as chitin, glucan, cellulose and proteins successfully limiting the growth of fungal pathogens [18,2]. As the skeleton of the fungal cell wall mainly contains chitin, glucan and proteins, mycoparasitism and enzymes that hydrolyze these components are one of the main mechanisms accounting for showing antagonistic activity against plant pathogenic fungi. Chitinase, glucanase and cellulase are important in the hyper-parasitic mechanism. Chitin and beta-1,3 glucan are the main structural components of fungal cell walls, except those from members of the class Oomycetes. Chitinase and glucanases produced by some Trichoderma sp. are the key enzymes in the lysis of cell walls during their mycoparasitic action against phytopathogenic fungi [5].
————————————————
• All other co-authors are also working in the same project.
Trichoderma species have been widely investigated as bio-control agents and are renowned to produce hydrolytic enzymes that act synergistically on plant and fungal cell wall polysaccharides. Enzymes from Trichoderma species, and esp. T. harzianum, have been used to degrade extracellular (1→3) (1→6)-β-D-glucans to produce gluco-oligosaccharides [8,3]. Cellulose is a major polysaccharide constituent of plant cell walls and one of the most abundant organic compounds on the earth. It is composed of β-
1,4-glucose units linked by β-1,4D-glycosidic bond, cellulose
degrading enzymes act by cleaving the glucosidic bonds [10].
Cellulases responsible for the hydrolysis of cellulose are
composed of complex mixture of enzyme. Cellulases are divided into three main classes [9]. These classes are endoglucanase (EC
3.2.1.4), Cellobiohydrolase (EC 3.2.191) and beta-glucosidase (3.2.121) [16]. Endogulacanase are generally called CMCases which generally attack randomly at 1,4 D- glycosidic bonds in cellulose.
The major goal of this research was to purify glucanase enzyme from the Trichioderma sp. In addition different biochemical properties of the isolated enzymes were also studied.
Trichoderma sp. previously isolated from the different
states of Uttar Pradesh were cultivated on Czapek Dox Medium containing CMC and wood dust as sole carbon source (1%). Cultures were incubated for 10-14 days on orbital shaker at 150 rpm. At the end of the incubation time contents of conical flasks were filtered and the filterate was centrifuged at 5000rpm for 10 min. The clear supernatant was considered as the source of crude enzymes [21].
Endoglucanase activity was routinely measured
according to [24]. The enzyme solution 1ml in appropriate dilution was added to 1ml of 1% carboxymethyl cellulose dissolved in 50Mm sodium acetate buffer, pH 5.0. After
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 647
ISSN 2229-5518
incubation at 50̊ C for 60 min. the reaction was stopped by the
addition of 3 ml DNS reagent. After incubating for 10 min. in a boiling water bath enzymatic activity was determined at 540 nm. One unit of CMCase activity was expressed as the amount of protein that liberate reducing sugar equivalent to glucose per minute under assay conditions.
Protein content of the crude enzyme preparation was
assayed by Lowry method [19], using BSA, as standard.
The clear supernatant used as a source of crude enzyme was
purified by the slow addition of Ammonium Persulfate with continuous stirring till 80% saturation. The obtained precipitate was dissolved in citrate phosphate buffer pH 5.0. Enzyme preparations were applied for FPLC treatment Sharp peak Fractions were collected and applied for SDS-PAGE analysis.
For molecular weight determination the enzyme preparation and
known molecular weight markers were subjected to electrophoresis according to Bollag and Edelstein [1] with 12% acrylamide gel. After electrophoresis gel was stained with Ezee blue gel stainer. Clear bands indicate the glucanase enzyme activity.
Thermal stability of enzyme was tested by preheating of enzymes
at 40̊, 50̊, 60̊ and 70̊C for 1hour.
The pH optima of glucanase enzyme were determined at pH
range from 2 to 9 using citrate phosphate buffer and tris buffer.
Effect of SDS and EDTA were determined at the concentration of
20µg/ml.
2.5
2
1.5
1
0.5
0
CMC Wood Dust
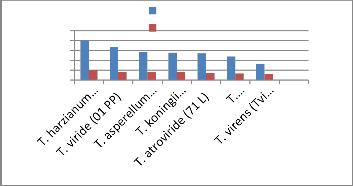
To achieve maximum production culture conditions were standardized. Two different carbon sources were added in the
culture media for maximum enzyme production. CMC was found to be the best glucanase inducer as compare to wood dust.

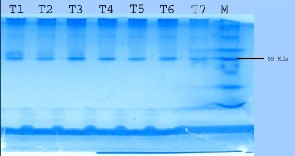
The molecular weight of the enzyme protein was calculated based on the basis of mobilities of the protein bands on SDS gel. Estimated molecular weight was 55 kda. Again the presence of single band under reducing and nonreducing conditions exhibits homogeneity of the enzyme. And from this it is evident that all the isolates have glucanase enzyme. El-Zawahry et al. [26] reported molecular weights of glucanase enzyme isolated from Trichoderma sp. around 55 kDa.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 648
ISSN 2229-5518
0.45
0.4
0.35
0.3
0.25
0.2
0.15
0.1
0.05
0
T. harzianum (Th azad)
T. viride (01PP) T. koningii (Tk (CSAU)) T. asperellum
(Tasp/CSAU)
T. virens (T.vi (CSAU)) T. atroviride (71 L) T. longibrachiatum
(21 PP)
(M) Molecular weight marker and (B) Activity pattern
Trichoderma samples for determination of molecular weight. From left to right,
Glucanases purified from the culture filtrate of Trichoderma sp. was assayed at different temperatures ranging from 30 to 70˚C and the optimal temperature was 50˚C. Enzymes show highest activity at 50˚C and their activity decreases beyond
50˚C. Kalra et al. [14] reported that the optimum temperature for
cellulolytic enzyme isolated from Trichoderma longibrachiatum was around 55˚C - 65 ˚C. Ulker and Spray [23] isolated low molecular weight endoglucanase from Trichoderma reesei with optimum temperature 52˚C.
SDS and EDTA have showed inhibitory effect on the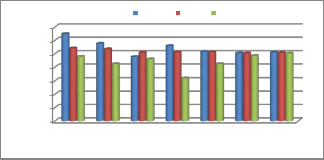
glucanase activity. EDTA is a chelating agent [24] and its inhibition ability indicates that specific ions might be actively involved in catalytic reaction of the enzyme [15].
0.700
0.600
0.500
0.400
0.300
0.200
0.100
0.000
T. harzianum (Th.
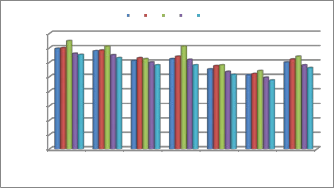
azad)
T. viride (01 PP) T. asperellum
(Tasp/CSAU)
T. koningii
(Tk(CSAU)
T. atroviride (71 L) T. longibrachiatum
(21PP)
T. virens (Tvi
(CSAU)
0.400
30ºC 40ºC 50ºC 60ºC 70ºC
0.350
0.300
0.250
0.200
0.150
0.100
0.050
0.000
T. harzianum
(Th. azad)
T. viride (01 PP) T. asperellum
(Tasp/CSAU)
T. koningii
(Tk(CSAU)
T. atroviride (71
L)
T. longibrachiatum (21PP)
T. virens (Tvi
(CSAU)
The major goal of this research was to identify the best carbon source for the induction of glucanase enzyme, viz. wood dust and
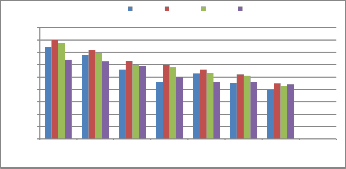
CMC under submerged fermentation conditions. Out of these tested carbon sources CMC was found to be the most effective carbon source for the induction of glucanase enzyme in seven isolates of Trichoderma. Out of these tested seven strains T. harzianum was found to be the most promising strain for glucanase enzyme production.
pH: To obtain maximum glucanase production by Trichoderma spp., each Erlenmeyer flask containing 50 mL growth media with pH ranging from 4.0-7.0 was incubated at 30oC with 2 mL inoculums for 8 days. After 8 days of incubation, glucanase activity was determined. Our results were confirmed by workers [20,22].
The isolated enzymes were run on SDS gel for the molecular mass determination and the molecular mass of the isolated enzymes were found in the range of 55 kDa.
The authors are grateful for the financial support granted
by the ICAR under the Niche Area of Excellence on “Exploration and Exploitation of Trichoderma as an antagonist against soil borne pathogens” running in Biocontrol Laboratory, Department of Plant Pathology, Chandra Shekhar Azad University of Agriculture and Technology, Kanpur, India.
[1]. Bollag, D. and S. Edelstein, 1991. Protein Methods. A John Wiley & Sons, Inc., Publication.
[2]. Carsolio C, Gutierrez A, Jimenez B, Van Montagu M &
Herrera-Estrella A (1994) Primary structure and
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 649
ISSN 2229-5518
expression pattern of the 33-kDa chitinase gene from the mycoparasitic fungus Trichoderma harzianum.
Proceading of the National Academy of Science, USA
91(23) , 10903–10907.
[3]. Chen LL, Zhang M, Zhang DH, Chen XL, Sun CY, et
al. (2009) Purification and enzymatic characterization of two β-endoxylanases from Trichoderma sp. K9301 and their actions in xylo-oligosaccharide production. Bioresour Technol 100: 5230-5236.
[4]. Chet I, Harman GE & Bake R (1981) Trichoderma hamatum, its hyphal interactions with Rhizoctonia solani and Phytium spp. Microbial Ecolog 7(1), 29–38.
[5]. de la Cruz J, Hidalgo-Gallego A, Lora JM, Benitez T,
Pintor-Toro JA & Llobell A (1992) Isolation and characterization of three chitinases from Trichoderma harzianum, European Journal of Biochemistry 206,
859–867.
[6]. Elad Y, Barak R, Chet I & Henis Y (1983) Ultrastructural studies of the interaction between Trichoderma spp. and plant pathogenic fungi, Phytopathology 107, 168–175.
[7]. Elad Y, Chet I, Boyle P & Henis Y (1983) Parasitism of Trichoderma spp. on Rhizoctonia solani and Sclerotium rolfsii-scanning electron microscopy and fluorescence microscopy. Phytopathology 73, 85–88.
[8]. Giese EC, Covizzi LG, Dekker RFH, Monteiro NK, Corradi da Silva ML, et al. (2006) Enzymatic hydrolysis of botryosphaeran and laminarin by β-1,3- glucanases produced by Botryosphaeria rhodina and Trichoderma harzianum Rifai. Process Biochem 41: 1265-1271.
[9]. Goyal, A., B. Ghosh and D. Eveleigh, 1991.
Characterisation of fungal cellulases. Biores. Technol.,
36: 37-50.
[10]. Harman GE, Hayes CK, Lorito M, Broadway
RM, Di Pietro A, Peterbauer CK & Tronsmo A (1993) Chitinolytic enzymes of Trichoderma harzianum: purification of chitobiosidase and endochitinase. Phytopathology 83, 313–318
[11]. Inbar J & I Chet (1992) Biomimics of fungal cell-cell recognition by use of lectin-coated nylon fibers, Journal of Bacteriology 174,1055–1059.
[12]. Inbar J & I Chet (1994) A newly isolated lectin
from the plant pathogenic fungus Sclerotium rolfsii: purification, characterization and role in mycoparasitism, Microbiology 140(3), 651–657.
[13]. Inbar J & I Chet (1995) The role of recognition
in the induction of specific chitinases during mycoparasitism by Trichoderma harzianum, Microbiology 141(11), 2823–2829.
[14]. Kalra, M., M. Sidhu, and D. Sandhu, 1986.
Partial purification, characterization and regulation of cellulolytic enzymes from Trichoderma
longibrachiatum. Journal of Applied Microbiology,
61(1): 73-80.
[15]. Kotchoni, O., W. Gachomo, B. Omafuvbe and
O. Shonukan,. Purification and Biochemical Characterization of Carboxymethyl Cellulose (CMCase) from a catabolite repression insensitive mutant of Bacillus pumilus. International Journal of Agriculture and Biology 2006., 8 (2): 286-292.
[16]. Krässig, H., 1993. Cellulose: Structure,
accessibility and reactivity. Gordon and Breach Science
Publishers S.A. 6-13: 187-205.
[17]. Lora JM, DeLa Cruz J, Benitez T & Pintor- Toro JA (1995) A putative catabolite-repressed cell wall protein from the mycoparasitic fungus Trichoderma harzianum , Molocular and General Genetics 247, 639-
645.
[18]. Lorito M, Harman CK, Di Pietro A, Woo SL
& Harman GE (1994) Purification, characterization and synergistic activity of a gulacan 1,3-beta glucosidase and an N-acetylglucosaminidase from Trichoderma harzianum, Phytopathology 84, 398-405.
[19]. Lowry OH, Rosebrough AL and Farr RJR, J.
Biol. Chem, 1951.,193-256.
[20]. Petrova, S., N. Bakalova and D. Kolev, 2009.
Properties of two endoglucanases from a mutant strain
Trichoderma sp. M7 with potential application in the paper. Applied Biochemistry and Microbiology, 45(2):
150-155.
[21]. Rajoka, M. and K. Malik, 1997. Cellulase
production by Cellulomonas biazotea cultured in media containing different cellulosic substrates. Bioresource Technology, 59(1): 21-27.
[22]. Sul, O., J. Kim, S. Park, Y. Jun Son, B. Park, D. Chung, C. Jeong, I. Han and 2004. Characterization and molecular cloning of a novel endoglucanase from Trichoderma sp. C-4. Appl Microbiol Biotechnol.,
66(1): 63-70.
[23]. Ülker, A. and B. Sprey, 1990. Characterization
of an unglycosylated low molecular weight 1,4-_- glucanglucanohydrolase of Trichoderma reesei. FEMS Microbiology Letters., 69(3): 215-219.
[24]. Miller, G., 1959. Use of dinitrosalicyclic acid
reagent for determination of reducing sugar. Anal. Chem. 31(3): 426-428.
[25]. Ali, S. and A. Sayed, 1992. Regulation of cellulase biosynthesis in Aspergillus terreus. World J. Microbiol. Biotechnol, 8(1): 73-75.
[26]. El-Zawahry,Y.A., El-Mougith,A.A, El-
Saadani, M.A, Hafez, E.E. and Soliman, S.A, 2010. Partial Purification and Characterization of Two Endo-
_-1, 4-glucanase from Trichoderma sp. (Shmosa tri), Australian Journal of Basic and Applied Sciences,
4(10): 4559-4568.
.
IJSER © 2014 http://www.ijser.org