International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 466
ISSN 2229-5518
Investigations of the effect of standard and potential anticancer drugs on the antioxidant activity of ascorbic acid
Munira Khalid, Rumana Qureshi and Afzal Shah*
Department of Chemistry, Quaid-i-Azam University, 45320, Islamabad, Pakistan
* To whom correspondence should be addressed
Tel: +92-5190642110
Fax: +92-5190642241
e-mail: afzals_qau@yahoo.com
Department of Chemistry
Quaid-i-Azam University, 45320, Islamabad, Pakistan.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 467
ISSN 2229-5518
Abstract
The influence of some potential and standard anticancer drugs on the antioxidant activity of ascorbic acid was examined by electrochemical and UV-visible spectroscopic techniques. The experimental results were complemented by theoretical calculations. The stoichiometry of the predominant complex formed between drugs and ascorbic acid was calculated by Job’s method. The antioxidant activity of ascorbic acid was estimated in terms of IC30 and IC50 by plotting a graph between radical scavenging activity and the concentration of the added ascorbic acid. Scavenging constant was calculated by using Bensei-Hilderbrand equation. IC30, IC50 and scavenging constants were also calculated from cyclic voltammetric data. AMI calculations using HyperChem were carried out to determine the binding energy and HOMO and LUMO energy values. An agreement was found between theoretical predictions
and experimental results.
studies; Cyclic voltammetry.
SER
Keywords: Antioxidant activity; Anticancer drugs; Scavenging constant; Computational
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 468
ISSN 2229-5518
1. Introduction
The use of antioxidant supplements by cancer patients is estimated between 13% and
87% [1-5]. The debate over the usefulness of and contraindications against antioxidants during anticancer therapy is currently based more on opinion than scientific fact. Patients may take antioxidant supplements while undergoing chemotherapy to help alleviate side effects from toxic chemotherapies and to improve the efficacy of chemotherapy. However, the use of antioxidant supplements by patients undergoing chemotherapy has been criticized due to concerns that antioxidants may interfere with the action mechanism of the chemotherapeutic agents and subsequently decrease their efficacy [6-8]. Others argue that antioxidant supplements are useful in conjunction with chemotherapy because these enhance the efficacy of chemotherapy, as well as alleviate toxic side effects, allowing patients to
[9-14].
IJSER
tolerate chemotherapy for the full course of treatment and possibly at higher doses. As a
result, patients may have better tumor response rates and increased chances of survival
One of the main mechanisms of chemotherapeutic drugs against cancer cells is the formation of reactive oxygen species, or free radicals. Some have argued that antioxidants scavenge the reactive oxygen species integral to the activity of certain chemotherapy drugs thereby diminishing the treatment efficacy. Others are of the view that antioxidants may mitigate toxicity and thus allow for uninterrupted treatment schedules and a reduced need for lowering chemotherapy doses [15-23]. The present work demonstrates the effect of some potential and standard anticancer drugs on the antioxidant activity of ascorbic acid using
cyclic voltammetric and UV-visible spectroscopic techniques.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 469
ISSN 2229-5518
2. Experimental
2.1. Materials and methods
Commercial anticancer drugs like daunorubicin, etoposoide, doxorubicin, cisplatin, fluorouracil and cyclophosphamide were used in the present work. Ascorbic acid purchased from Sigma was used as antioxidant. Stock solutions of the compounds were prepared in analytical grade methanol.
Molecular modeling for the charge-transfer complexes were performed by using Hyper- Chem. Release 07 software. After geometric optimization of the drugs and building their molecular structures single point energy calculations were done with the objective of finding theoretical orbitals and binding energies from AM1 calculations. The structures of the drugs were then merged one by one with antioxidant and their binding energies were determined. The binding energy difference of drugs before and after merging was calculated for the
theoretical prediction of complex formation. The results are shown in table 1.
The antioxidant activity of ascorbic acid was determined by UV-visible spectroscopy in the absence and presence of anticancer drugs. The spectrum of 80 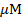 solution of DPPH•
solution of DPPH•
was recorded and different concentrations of ascorbic acid were added gradually to check the scavenging ability of ascorbic acid. The effect of anticancer drugs on ascorbic acid was also studied. The IC50 values were obtained by calculating the radical scavenging activity through the following relation [24]
%RSA = 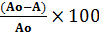 (1)
(1)
Mixtures of ascorbic acid and anticancer drugs were prepared and their scavenging effect was noticed by adding microliters of mixture to DPPH• solution with the help of micropipette. The scavenging constant was calculated from the following relation [25]
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 470
ISSN 2229-5518
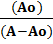 =
= 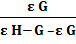 +
+ 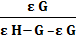
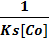 (2)
(2)
The interacting ratio of drug and ascorbic acid was deduced from Job’s method. For this method a series of solutions of the drugs and ascorbic acid ranging from 0 to 1 mole fractions were prepared. The spectra of the mixtures were recorded and Job’s plot was obtained by plotting absorbance versus mole fraction of ascorbic acid. A maxima was obtained in the graph at a ratio corresponding to the predominant complex formation. Most of the drugs formed complexes with ascorbic acid in equimolar ratio i.e. 1:1 while only two complexes formed in 1:2 ratio and 2:1 ratio.
Cyclic voltammetric investigations were conducted using conventional three electrode system of glassy carbon, saturated calomel and thin platinum wire acting as working, reference and counter electrodes respectively. The concentration of DPPH• solution used was
these solutions were prepared in methanol. The potential range for DPPH• was chosen
5 mM. 0.1 M tetrabutylammonium perchlorate was used as supporting electrolyte. Both of
between -0.2 to +0.6 V. The same procedure as proceeded in UV studies was employed in cyclic voltammetry. The antioxidant activity of ascorbic acid was measured by adding microliters of antioxidant to DPPH• solution with the help of micropipette and voltammogram was recorded that witnessed a decrease in peak current value. The concentration of ascorbic acid was varied from 40 µM to 200 µM. IC50 values were obtained by using the following relation
%RSA = 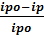 × 100 (3)
× 100 (3)
Then different concentrations of ascorbic acid-anticancer drugs mixture were prepared and added to DPPH• solution. The variation in the behavior of peak current of DPPH• with the
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 471
ISSN 2229-5518
addition of ascorbic acid was noticed. The scan rate used throughout the experiments was 100 mVs-1. The scavenging constant was calculated from voltammetric studies by using the relation [26]
log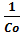 = logKscav + log
= logKscav + log 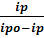 (4)
(4)
3. Results and discussion
3.1. Theoretical calculations
Possible drug interactions with ascorbic acid were predicted using AM1 calculations software. Based on the energies of HOMO and LUMO of drugs, relative electron pair donating or accepting properties were predicted. Charge transfers from one drug to the other if LUMO of acceptor drug stabilize the electron pair donated by the donor drug. The results of semi-empirical calculations can be seen in table 1. Based on the binding energy difference
form stable complexes with ascorbic acid. Generally molecules with more negative values of
(ΔB.E) it was predicted that all of the investigated anticancer drugs except doxorubicin will
EHOMO do not act as donors as the outer electrons are too tightly bound whereas, molecules with less negative EHOMO can act as donors. Conversely the molecules with negative E LUMO values act as acceptors as negative value of energy implies that such molecules will stabilize the incoming electron. Comparing the energies of HOMO of the standard anticancer drugs with that of ascorbic acid it can be predicted that the drugs doxorubicin, daunorubicin and etoposoide will act as donors towards ascorbic acid. This implies that HOMO of drug would interact with LUMO of ascorbic acid [27]. The HOMO of drugs and LUMO of ascorbic acid have been depicted in fig.1. The other three standard drugs i.e. cisplatin, fluorouracil and cyclophosphamide cannot act as donors hence they will play the role of acceptors as their ELUMO have more negative values. The ELUMO and EHOMO of ascorbic acid along with merged
structures are shown in fig 2. All four potential anticancer drugs will act as donors as their
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 472
ISSN 2229-5518
EHOMO are less negative as compared to ascorbic acid. The EHOMO and ELUMO of all drugs are listed in table 1.
3.2. UV-Visible spectroscopic measurements
3.2.1 Characterization of ascorbic acid and DPPH
Under the specified optimum reaction conditions, the calibration curves of ascorbic acid and DPPH radical were recorded. The electronic absorption spectra of DPPH and ascorbic acid have been presented in fig. 3. Their peaks at 516 nm and 244 nm were used for the evaluation of extinction coefficients.
The possibility of drug-ascorbic acid adduct formation was investigated by UV-visible spectroscopy. In case of interaction the molar ratio of the anticancer drug-ascorbic acid adduct could be determined by using method of continuous variation i.e., Job’s method [28]. For this purpose a number of solutions of ascorbic acid and drugs were prepared by varying
concentrations and a graph was plotted between mole fraction and absorbance. The maximum
their mole fraction. The electronic absorption spectra were obtained for all the prepared
in absorbance was obtained at composition corresponding to the stoichiometry of predominant complex. The job’s plot is shown in fig. 4. It can be seen that most of the drugs interact with ascorbic acid in 1:1 ratio while daunorubicin and fluorouracil combine with ascorbic acid is 1:2 and 2:1.
3.2.2 Scavenging of DPPH radical by mixture of ascorbic acid and anticancer drugs
UV-visible spectra of DPPH• in the presence of drugs were taken to see whether the drug itself has any scavenging capacity, but none of the drug alone showed any quenching. The influence of doxorubicin on the spectrum of pure DPPH• was examined and the results showed no effect. The same behavior was observed for all drugs implying that none of these drugs have any antioxidant activity.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 473
ISSN 2229-5518
In order to investigate the effect of anticancer drugs on the antioxidant activity of ascorbic acid the response of DPPH• was recorded in the presence of mixture of ascorbic acid and drug. The peak height of DPPH• was found to decrease on the addition of mixture. IC50 values were calculated for these mixtures of ascorbic acid and anticancer drugs and compared
to that of pure ascorbic acid. The increase in IC50 value in the presence of anticancer drugs- ascorbic acid mixture indicated a decrease in the antioxidant activity of ascorbic acid. The pictorial representation in connection with doxorubicin can be seen in fig. 5. All other drugs followed the same trend. The values of IC50 and percentage change in IC50 are given in tables 2 and 3.
3.2.3 Scavenging effect of ascorbic acid and ascorbic acid-drug adduct
The interaction study between ascorbic acid and ascorbic acid-drug adduct was measured by
IJΔG= -RTlSnkscav
ER (5)
calculating the scavenging constant using Benesi-Hildebrand equation as given in Eq. 2.
Gibbs free energy of the reaction was also calculated by employing the equation
The values of scavenging constant and ΔG are presented in tables 4 and 5. The scavenging constants complement IC50 values. Since the IC5O values suggest that antioxidant activity of ascorbic acid is reduced in the presence of anticancer drugs, the scavenging constants also complement those results because scavenging constant of ascorbic acid is also decreased in the presence of a drug which means that antioxidant capacity is mitigated.
3.3. Electrochemical measurements
The effect of anticancer drugs on the antioxidant activity of ascorbic acid was also experimentally probed by cyclic voltammetry. Before analyzing the effect on antioxidant activity the electrochemical behavior of DPPH• was studied. The effect of the addition of ascorbic acid and ascorbic acid-drug adduct on the DPPH˙ was examined by cyclic
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 474
ISSN 2229-5518
voltammetric analysis (Fig. 6). By the addition of ascorbic acid alone a sharp decrease in peak current was observed. As the ascorbic acid-drug adduct was added to the DPPH• a decrease in peak current was also witnessed. This decrease was smaller as compared to that for pure ascorbic acid. IC50 and scavenging constant values were evaluated from electrochemical analysis.
4. Conclusions
The effect of six standard and four potential anticancer drugs on the antioxidant potential of ascorbic acid was studied by employing UV-visible spectroscopy and cyclic voltammetry. The experimentally obtained results were correlated to the theoretical AM1 calculations. The effect was more significant in case of etoposoide, cisplatin, daunorubicin, fluorouracil and
cyclophosphamiIde, buJt negligibSle effect was Efound in caseRof doxorubicin. Both cyclic
voltammetric and UV-Visible spectroscopic techniques predicted that the effect of etoposoide
on the antioxidant activity was maximum whereas for doxorubicin it was minimum. Although the magnitude of the percentage change in IC50 of ascorbic acid on addition of drug was not the same from both experimental techniques but the general trend observed was the same. It was also found that the potential anticancer compounds, which are substituted benzimidazoles, affected the antioxidant activity of ascorbic acid to a greater extent and the same trend was revealed by the results obtained from cyclic voltammetry and UV-visible spectroscopy.
By carrying out AM1 calculations binding energy values for ascorbic acid-drug adduct were calculated and it was found that adducts giving negative value for binding energy exhibit interactions on experimental investigations. A positive binding energy difference for doxorubicin implying no interaction was complemented by the experimental
results where doxorubicin did not affect the antioxidant activity of ascorbic acid. Similarly
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 475
ISSN 2229-5518
HOMO and LUMO energy values for adducts were also calculated and it was found that theoretical results suggest the complex formation in all cases except doxorubicin. Thus, the theoretical calculations predicted the same behavior as revealed by experimental studies.
Acknowledgments
We acknowledge Quaid-i-Azam University Islamabad for the provision of funds and experimental facilities.
IJSER
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 476
ISSN 2229-5518
References
[1] Becker E. M.; Nissen L.R.; Skibsted L. H. European Food Research and Technology
2004, 219, 561-571.
[2] Fenton, H. J. H. Journal of the Chemical Society 1894, 65, 899-910.
[3] Amatore C.; Arbault S.; Koh A.C.W. Analytical. Chemistry, .2010, 82, 1411-1419.
[4] Amatore C.; Arbault S.; Bouton C.; Drapier J.C.; Ghandour H.; Koh A.C.W. Chem
BioChem 2008, 9, 1472- 1480.
[5] Benov L.; Sztejnberg L.; Fridovich I. Free Radical Biology & Medicine 1998, 25,
826-831.
[6] Litescu SC.; Sandra AV.; Eremia SAV.; Diaconu M.; Tache A. Annals of Biological
[7] Prasad K.N.; Kumar A.; Kochupillai V.; Cole W.C. Journal of the American College
Research 2011, 11, 35-37.
of Nutrition. 1999, 18, 13-25.
[8] Halliwell, B.; Whiteman, M. British Journal of Pharmacology .2004, 142, 231-255. [9] Stocker, R.; Frei, B. Oxid. Stress, Academic: 1991, 213-243.
[10] A, C. D. P., Cahiers de nutrition et de diététique 1991, 26, 2, 137-143. [11] Blois, M.S. Nature 1958 181, 1199-1200.
[12] Pincemail, J.; Deby, C.; Dethier, A.; Bertrand, Y.; Lismonde, M.; Lamy, M.
Bioelectrochemistry and Bioenergetics 1987, 18, 117-126.
[13] Block, G. Nutrition Reviews 1992, 50, 7, 207-213.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 477
ISSN 2229-5518
[14] Burton, G. W.; Ingold, K. U. Nutrition Reviews Science 1984, 224, 4649, 569-573.
[15] Agnete, E.K.; Kristoffersen, M.A.; Vinjar, F. The journal of alternative and
Complementary Medicine. 2008, 14, 911-919.
[16] Boon H.; Stewart M.; Kennard MA. J Clin Oncol 2000 , 18, 2515-2521. [17] Omenn GS.; Goodman G.; Thomquist M. Cancer Res 1994 ,54, 2038-2043. [18] Geert V P.; Henk V.D Berg. Cancer letters 1997,114, 195-202.
[19] Davis, W.; Lamson, M.S .; Matthew S.; Brignall, ND. Altern Med Rev 1999, 4, 5,304-
329.
[20] Keith I .Block.; Amanda C. Koch .; Mark N. Mead .; Peter K. Tothy.; Robert A.
179.
IJSER
Cancer Treatment Reviews 2007, 33, 407-418.
[21] S,K Myung.; Y. Kim.;W. Ju.; H.J.Choi.; W.K.Bae. Annals of oncology 2010 21, 166-
[22] S.M Sagar . Current oncology. 12, 2, 44-54.
[23] Thomas E.I.; Boris M.; Todd B.; Brandon L.; Ron H.; Nina A.M.; James A.J.; Michael J.G.; Jorge R.M.; Doru T.A.; Constantin A.D.; Vladimir B.; Janis A.; Brian S.; Boris M.; James K.; Chien-Shing C.; Neil H.R. Journal of translational medicine
2011, 9, 1-13.
[24] Barros L.; Falco S.; Baptista P.; Freire C.; Vilas-Boas M.; Ferreira I.C.F.R. Food and
Chemical Toxicology, 2008, 46, 2742-2747.
[25] Hiegmann M.; Scholz F.; Kahlert H.; Caravalho L.M.D.; Rosa M.B.D.; Lindequist U.; Wrust M.; Nascimento P.C.D.; Boher D. Electroanal. 2010, 22, 46.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 478
ISSN 2229-5518
[26] Yang G.J.; Xu, J.J.; Chen H.Y.; Leng Z.Z. Chin. J. Chem. 2004, 22, 1325.
[27] Lewars E.; Computational Chemistry, Introduction to the Theory & Application of
Molecular Mechanics, Kuluwar Academic Publishers, London, 2003.
[28] Perkampus H-H, UV-Vis spectroscopy and its applications springer- verlag 157-158
IJSER
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 479
ISSN 2229-5518
Tables
Table 1. Molecular orbital energies and binding energy differences of the investigated drugs.

Category Drug Acronym
used
EHOMO
(ev)
ELUMO
(ev)
ΔB.E
(ev)
Antioxidant Ascorbic acid AA -9.68 -0.53 ---
Doxorubicin Doxo -8.12 -1.11 0.09
Flourouracil Flouro -9.81 -0.65 -1.63
Standard drugs
Cyclophosphamide Cyp -10.03 -1.30 -0.7
Cisplatin Cptn -11.1 -0.48 -1.1
IJDaunorubicSin DauEno -8R.21 -1.13 -0.11
Etoposoide Etopo -8.54 -0.06 -0.1
2(4-butoxyphenyl)-
5-nitro-2,3-dihydro-
1H-indene
BMP -9.05 -0.42 -1.09
Potential drugs
–5-nitro-2-(1H- pyrrol-2-yl)-1H- benzimidazole
BMN -9.10 -1.77 -1.98
2-(5-methylfuran 2- yl)-5-nitro-1H- benzimidazole
BMO -9.65 -0.88 -3.69
5-nitro-2-(thiophen-

2-yl)-1H- benzimidazole
BMS -9.15 -0.47 -0.69
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 480
ISSN 2229-5518
Table 2. UV-visible spectroscopically determined IC50 values of ascorbic acid & mixture of ascorbic acid and standard anticancer drugs.

IJDoxorubicSin+AA
E1.23E-6
R2.5

IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 481
ISSN 2229-5518

Table 3. UV-visible spectroscopically determined IC50 values of ascorbic acid & mixture of ascorbic acid and potential anticancer drugs.
Compound | IC50 (M) | %age change in IC50 * |
Pure AA | 1.2E-6 | ---- |
BMP - AA | 2.66E-6 | 116 |
BMO - AA | 2.7E-6 | 125 |
BMN - AA | 3.09E-6 | 157 |
BMS - AA | 3.8E-6 | 260 |

Table 4. Scavenging constant and ∆G of ascorbic acid-standard anticancer drug mixture with DPPH∙ as determined by UV-visible spectroscopy.

Compounds | Scavenging constant(M-1) | ∆G(kJ/mol) |
Pure AA | 9.0E4 | -25.90 |
Doxorubicin + AA | 8.89E4 | -25.86 |
Fluorouracil + AA | 2.5E4 | -22.98 |
Cyclophosphamide + AA | 1.8E4 | -22.23 |
Cisplatin + AA | 7.4E4 | -25.44 |

IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 482
ISSN 2229-5518

Etoposoide + AA 2.9E4 -23.32
Daunorubicin + AA 3.4E4 -23.68

Table 5: Scavenging constant and ∆G of ascorbic acid-potential anticancer drug mixture with DPPH∙ as determined by UV-visible spectroscopy.

IBMOJ- AA
S1.07EE3
R-15.83

IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 483
ISSN 2229-5518
Figures
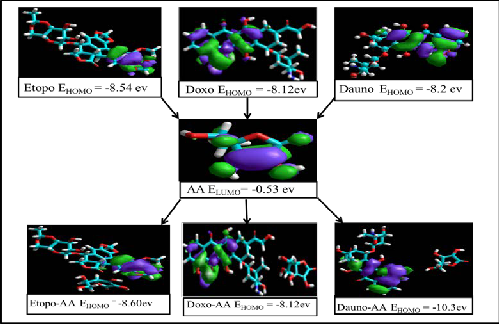
IJScompleExes. R
Fig. 1. EHOMO of ascorbic acid, daunorubicin, etoposoide and doxorubicin and their predicted
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 484
ISSN 2229-5518
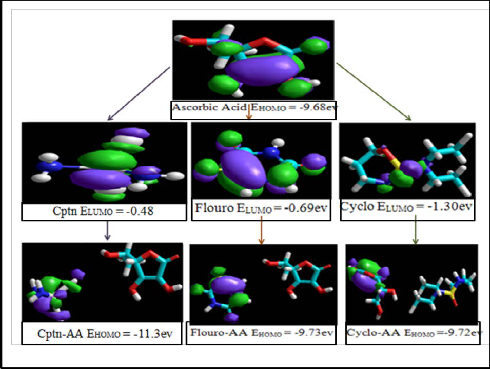
Fig. 2. ELUMO of ascorbic acid, cisplatin, fourouracil and cyclophosphamide and their
IJSpredicted coEmplexes. R
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 485
ISSN 2229-5518
λ 515 nm
0.60 A
max
0.45
0.30
0.15
0.00
400 500 600 700 800
λ (nm)

λ 244 nm
max
0.6
0.2
B
SER
0.4
0.0
-0.2
200 300 400 500
λ (nm)
Fig. 3. Electronic absorption spectra of (A) pure DPPH radical
and (B) ascorbic acid.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 486
ISSN 2229-5518
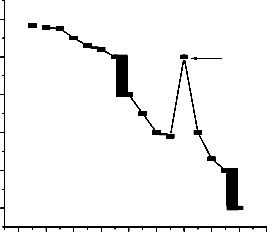
1.2 A
1.1
1 : 2
1.0
0.9
0.8
0.7
IJSMole fracEtion of AA R
0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0
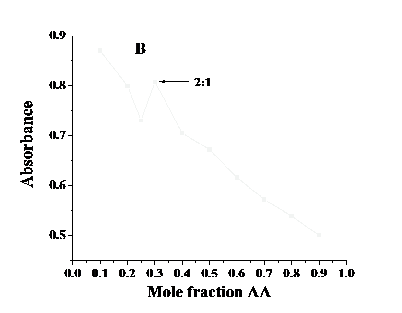
Fig. 4. Job’s plots for (A) daunorubicin
and (B) fluorouracil.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 487
ISSN 2229-5518
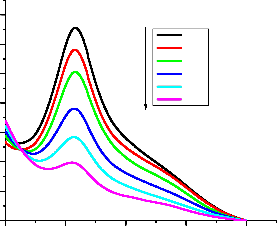
1.4
1.2
1.0
0.8
[Doxo-AA]µΜ
0.00
0.66
1.33
2.00
2.66
3.33
IJ0.4 SER
0.2
0.0
400 500 600 700 800
Wavelength(nm)
Fig. 5. UV-visible spectra of DPPH˙ in the absence and presence of ascorbic acid –
doxorubicin adduct.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 488
ISSN 2229-5518
30.0µ
20.0µ
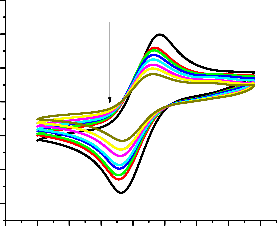
[Doxo-AA]µM
10.0µ
0.0
-10.0µ
I-2J0.0µ SER
-30.0µ
-0.3 -0.2 -0.1 0.0 0.1 0.2 0.3 0.4 0.5
E (V)
Fig. 6. Cyclic voltammograms of DPPH∙ in the absence and presence of doxorubicin-ascorbic acid adduct.
IJSER © 2013 http://www.ijser.org







