International Journal of Scientific & Engineering Research Volume 4, Issue3, March-2013 1
ISSN 2229-5518
Investigation of the Parameters Affecting Castor Oil Transesterification Reaction Kinetics for Biodiesel Production.
Aldo Okullo Apita and Abraham K. Temu
Abstract - Kinetic studies are concerned with the quantitative description of how fast chemical reactions take place and factors affect- ing them. The study is important to a chemist to understand the fundamental aspects of the reaction pathways to achieve the desired product. It enables us to tailor a chemical reaction so as to produce the desired product in a controlled manner. Castor oil was trans- esterified with methanol using a molar ratio of methanol to oil (6:1) and sodium hydroxide catalyst (1% wt of oil). The product of the reaction is castor methyl ester (CME) and glycerol with diglyceride and monoglyceride as intermediate products. The experiment was set to determine the effect of temperature, stirring rate and residence time on the rate constants and a second order rate law was used. Four different temperatures (35, 45, 55 and 65oC) and four stirring rates (600, 660, 700 and 800 rpm) were used and the reac- tions were timed. High temperatures (55 and 65oC) were unfavourable for castor transesterification; both temperatures provided yields of 91%. Low temperatures (35 and 45oC) increased rate constants and produced highest yields (98% and 97% respectively). Increas- ing stirring rate does not favour conversion rates. The optimum reaction time was 60 minutes. Beyond 90 minutes, the reverse reac- tion was favoured. The close fit of the data indicates that the selected model was adequate. These results agree well with reports from
literature.
Key Words: Castor Methyl Ester, Castor Oil, Kinetics, Rate Constants, Second Order Rates, Transesterification.
—————————— ——————————
1 INTRODUCTION
HE use of vegetable oils and derivatives as alternative diesel fuels and blends has become increasingly important over the past few years. The European Union Directive
2003/30/EC mandating the use of 2 and 5.75% biofuels for all petrol and diesel used in transportation by 2005 and 2010 re- spectively and EU 2005/93/EC allowing exemptions on excise duties on biofuels together with the US Energy policy (EPAct.
1992) all have contributed to the acceleration of the use of al- terative fuels for transportation [1].
Biodiesel is a renewable, biodegradable fuel made from vegetable oils and animal fats. A viable commercial method of production that has been used is alkaline transesterification of the oil or fats with alcohol. Methanol has been the most pre- ferred alcohol due to its low cost and short chain that makes it dissolve the catalyst easily. Sodium hydroxide is the common- ly used catalyst because it is cheaper and easily available than the other alkaline catalysts. The product of the reaction is me- thyl ester (biodiesel) and glycerol, with intermediate com- pounds of monoglyceride and diglyceride.

Castor beans plant (Palma Christi) belongs to the Euphor- biaceae family. It is drought resistant and can grow on mar- ginal lands. It is among the plants with high yield potential though its average yield is 1413 L/ha [2], [3].
Aldo Okullo Apita is currently pursuing PhD degree pro- gram in Chemical Engineering in Dar es Salaam Univesrity, Tanzania, PH-256772611967, E-mail: aldoapita@gmail.com
Abraham K. Temu is currently a Senior Lecturer in Dar es
Salaam University, Tanzania, PH-255754865791, E-mail:
atemu8@yahoo.co.uk
Castor has high oil content up to 56% of the seed; however, the oil is not used in food chain due to the toxin known as castor beans allergen (CBA) found in its seeds [2]. Its oil favours transesterification with minimum heating and stirring since it is soluble in alcohol [3].
Varma and Madras [4], among others synthesised methyl ester from castor oil and lindseed oil in supercritical fluids to determine the effects of molar ratio and temperature on the yield. However, data on parameters that affect castor oil trans- esterification is still scarce. In this study, castor oil was trans- esterified with methanol and sodium hydroxide as a catalyst. The experiment was set to determine the effect of temperature, mixing intensity and reaction time on rate of reaction of castor oil. Second order rate law was used to determine rate con- stants from the following consecutive and reversible reaction:
k1
TG + ROH  DG + RCO2R1 (1)
DG + RCO2R1 (1)
k2

k3

DG + ROH MG + RCO2R2 (2)
k4

k5

MG + ROH GL + RCO2R3 (3)
k6
Where: TG = triglycerides; DG = diglyceride; MG = mono- glyceride; GL = glycerine; ROH = methanol and RCO2R1,
RCO2R2, RCO2R3, are methyl esters; k1, k3, k5 are forward rate constants and k2, k4 and k6 are reverse rate constants.
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue3, March-2013 2
ISSN 2229-5518
2 MATERIALS AND METHODS
2.1 Materials
Castor beans were obtained from Dodoma region in Tan- zania. Analytical grade sodium hydroxide, methanol (99% purity) and potassium hydroxide (98% min. assay) were local- ly obtained from Dar es Salaam. GC calibration standards were obtained from Choice Analytical Pty, Thornig, Australia.
2.2 Methods
Transesterification was carried out in a two-litre round bot- tom flask immersed in a constant temperature water bath. The flask was fitted with a stirrer, a condenser, a thermometer and a sampling port. A catalyst (1% wt of oil) was dissolved in the required amount of methanol and the solution was added on to the oil in the reactor while stirring started immediately. This was the starting time for the reaction which was allowed to continue for two hours.
Samples were drawn at five minutes interval initially for 20 minutes then changed to 10 minutes intervals. These were quenched in a mixture of two ml tetrahudrofuran and two ml
0.5M sulphuric acid. Samples were shaken and centrifuged at
2000 rpm for 15 minutes to separate the methyl ester from the
glycerine. The methyl ester was washed with warm water
(50oC) three times and dried using anhydrous sodium sul-
phate and kept for analysis below minus two degrees celsius.
A GC/FID Shimadzu 2010 model A17, AAF V3 was used for
the quantification of TG, DG, MG, GL and the fatty acid me-
thyl ester (FAME). Gas Chromatographic analysis was carried out according to the ASTM D 6584/EN14105 standards.
3 RESULTS AND DISCUSSIONS
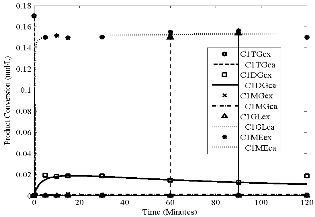
Figs 1 to 4 show the conversion of castor oil into castor me- thyl ester (CME) for different temperatures. The results for all these temperatures show that the reaction was complete with- in 90 minutes instead of 120 minutes as reported for other oils (points indicated by solid arrows in Figs. 1- 4). The highest conversions appeared after 30 and 60 minutes of the reaction (points indicated by dash arrows). Longer reaction time than
90 minutes resulted into the formation of the reactants as indi- cated by the reversibility of the reaction (1 – 3). This is due to high solubility of castor oil in methanol, in the product CME formed and in glycerine. The curves also show characteristics of consecutive and reversible reactions; whenever there is an increase of methyl ester, there is a subsequent decrease of di- glyceride or monoglyceride. This supports the proposed se- cond order model for the reaction and the close fit of the data indicates that the model was adequate.
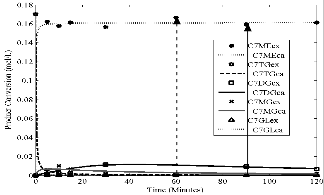
Fig1.eps, Conversion versus time. Temperature = 35oC, stirring rate = 700 rpm; molar ratio = 6:1 and catalyst loading = 1%
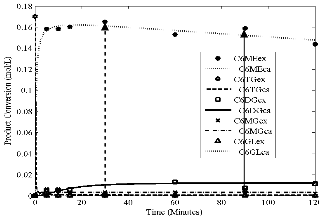
Fig2.eps, Conversion versus time. Temperature = 45oC, stirring rate = 700 rpm; molar ratio = 6:1 and catalyst loading = 1%
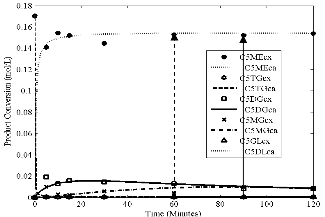
Fig3.eps, Conversion versus time. Temperature = 55oC, stirring rate =
700 rpm; molar ratio = 6:1 and catalyst loading = 1%
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue3, March-2013 3
ISSN 2229-5518
100
80
60
a b c
40
20 35oC 45oC 55oC 65oC
0
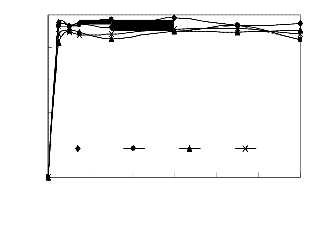
Fig4.eps, Conversion versus time. Temperature = 65oC, stirring rate
= 700 rpm; molar ratio = 6:1 and catalyst loading = 1%
3.1 Temperature Effect
Figure 5 shows temperature effects on the transesterifica- tion of castor oil. It is not easy to see a straight forward trend of the effect of temperature on the conversion of castor oil to methyl ester. However, low temperature favours conversion; the highest conversion of 98% was observed for a temperature of 35oC after 60 minutes of reaction whereas the second high- est conversion of 97% was seen for a temperature of 45oC after only 30 minutes. Temperatures of 55 and 65oC gave lower conversions of 90 and 91% respectively compared to the pre- vious ones (Fig 5). Castor oil has a behaviour which is differ- ent from other vegetable oils due to the presence of three func- tional groups in its structure; firstly it has 90% ricinoleic acid with hydroxyl group attached to the 12-carbon atom, secondly it has a double bond on the 9th carbon atom and thirdly it has a carbonyl (carboxylic) group on the first carbon atom. This structure favours solubility and low temperature operability of castor oil. Meneghetti [5], reported maximum yield of CME in one hour after which reversibility became more prominent. Da Silva [6], reported low temperature transesterification of castor oil to the methyl ester while [7] reported optimum transesterification temperature of castor oil as being 40oC and optimum time of reaction of 90 minutes. High solubility of castor oil in methanol was also reported by [2] giving a trans- esterification period of 30 minutes. The swinging of points at (a), (b) and (c) show the reversible nature of the reaction; point (a) reveals a high conversion after 30 minutes for a reaction at
45oC while point (b) shows a high conversion after 60 minutes for a reaction at 35oC and at point (c), all reactions give almost the same conversion after 90 minutes.
0 20 40 60 80 100 120
T ime (min)
Fig5.ps, Temperature Effect on CME conversion
3.2 Stirring Effect
There is no clear trend in mixing intensity effect on the
conversion of castor oil. High mixing intensity of 800 rpm
tends to favour the reversed reaction with the formation of
reactants. The solubility of CME in methanol and in glycerine
makes the reverse reaction possible under high temperature
(55oC) at this impeller speed, especially after one hour when
CME is sufficiently formed (Fig 6). Increasing mixing intensity
with castor oil transesterification has no advantage and there-
fore not necessary. Similarly, the reversible nature of the reac-
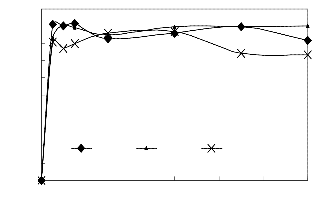
tion is shown in Fig 6 by the swinging of points.
100
90
80
70
60
50
40
30
20 600 rpm 700 rpm 800 rpm
10
0
0 20 40 60 80 100 120
Time (min)
Fig6.ps, Stirring effect on CME conversion
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue3, March-2013 4
ISSN 2229-5518
3.3 Rate Constants and Activation Energies


Rate constants and activation energies were determined for castor oil transesterification and the results are given in Table 1. Rates were lower at higher temperatures. Fast for- ward reactions; (TG DG), (DG MG) and (MG GL) were high at low temperatures. The reactions eventually slowed down at high temperatures. The rate determining step was the conversion of monoglyceride to glycerol (k5) for the entire period of the reaction. The higher concentra- tions of DG than MG as seen in the plots were expected since the second reversible reactions (k4) was faster than the third forward (k5) at high temperatures (Table 1). This shows that high temperature favours the reversible reac- tion. The explanation still lies in the structure of the triglyc- erides with three functional groups that make castor oil operate under low temperatures.
TABLE 1

RATE CONSTANTS AND ACTIVATION ENERGIES OF CASTOR CME
35oC 45oC 55oC 65oC Ea R2
(Jmol-1)

k1 0.9685 0.0302 0.0020 0.0002 236.2954 0.994 k2 0.0048 0.0042 0.0020 0.0017 33.6924 0.912 k3 0.2371 0.0187 0.0015 0.0003 190.5803 0.988 k4 0.0334 0.0031 0.0005 0.0001 172.5793 0.996 k5 0.1075 0.0018 0.0001 0.0000 304.3702 0.997 k6 0.1620 0.0082 0.0011 0.0001 209.9125 0.990
SSE 0.00007 0.00003 0.00004 0.00003

3.4 Castor Methy Ester Quality

The amounts of triglycerides (TG), diglyceride (DG), monoglyceride (MG) and glycerine (GL) remaining in the product methyl ester after purification are out of the range specified in EN14214 standard (Table 2). Since conversions are relatively high, this may indicate an insufficient purification of the methyl ester. However, repeatability is within the range of specification except for DG and hence total glycerides. Wash- ing methyl ester with water alone does not produce sufficient separation of CME from glycerine. CME and glycerol associate through hydrogen bonding. They form a homogeneous phase in methanol which is not easy to separate. A better method of separation for such heat sensitive compounds like CME would have been molecular distillation where a liquid component evaporates without boiling due to high vacuum.
TABLE 2
PRODUCT COMPOSITION SHOWING CME, FREE AND TOTAL GLYCERINES IN THE MIXTURE
4 CONCLUSIONS
Increasing temperature did not favour the rate of reaction of castor oil; this behaviour was attributed to castor oil’s 4. triglyceride structure with three functional groups on the mol- ecule. Increasing mixing intensity is not necessary since the oil is soluble in methanol.
The optimum transesterification conditions for castor oil were: temperature, 35oC; reaction time, 60 minutes; molar ratio of 6:1 methanol to oil and moderate stirring (600 rpm). A product yield of 98% methyl ester was obtained under these conditions. It was observed that a reaction time longer than 90 minutes results in reactants formation due to the reversibility of the reaction.
Separation of castor methyl ester from glycerol requires molecular distillation since the methyl ester is soluble both in methanol and in glycerol. The close fits of the calculated data to the experimental data points in this study showed that the model selected for the study was adequate. Castor oil trans- esterification can be described by second order rate law.
Castor methyl ester is not suited for use in diesel engine due to its high kinematic viscosity. Alternative method of re- ducing its viscosity should be looked into; such as blending with petro-diesel or with some other appropriate additives.
ACKNOWLEDGMENT
We would like to thank the Committee of Research and Publications of Kyambogo University and Alternative Energy for Sustainable Development Environmental Protection and Poverty Reduction in Tanzania (ESEPRIT) Project coordinator for the financial support during the realisation of this paper.
REFERENCES
[1] Bautista Luis Fernando, Gemma Vicente, Rosalia Rodriguez, Maria Pacheco, “Optimization of FAME production from waste cooking oil for biodiesel use”, Biomass and Bioenergy vol. 33, pp. 862-872, 2009.
[2] Encinar J. M., Gonzalez J. F., Martinez G., Sanchez N. and Gonzalez C.
G., “Synthesis and Characterization of biodiesel obtained from castor oil transesterification”, International Conference on Renewable Ener- gies and Power Quality (ICREPQ’11). Las Palmas de Gran Canaria (Spain), 13th to 15th April 2010.
[3] Bello E. I. and Makanju A., “Production, Characterization and Evalua- tion of Castor oil Biodiesel as Alternative Fuel for Diesel Engines”, Journal of Emerging Trends in Engineering and Applied Sciences (JE- TEAS), vol. 2, no. 3, pp. 525-530, 2011.
[4] Varma Mahesh N., and Madras Giridhar, “Synthesis of Biodiesel from
GLS
(%m/m) 35oC 45oC 55oC 65oC
EN1421
4
Castor Oil and Linseed Oil in Supercritical Fluids”, Ind. Eng. Chem. Res. Vol. 46, no. 1-6, 2007.
GL 0.14 0.13 0.14 0.16 ≤ 0.02
R 0.01±0.00 0.01±0.00 0.01±0.00 0.01±0.00
MG 1.11 1.23 4.20 0.67 ≤ 0.80
R -0.03±0.05 -0.03±0.02 -0.06±0.05 0.02±0.01
DG 3.86 13.68 7.75 10.96 ≤ 0.20
R 0.52±0.48 0.78±0.46 1.46±0.45 1.94±0.29
TG 6.35 1.97 1.64 2.66 ≤ 0.20
R 0.41±0.06 0.34±0.22 0.20±0.04 0.22±0.04
T.GL 11.46 17.00 13.73 14.44 ≤ 0.25
R 1.92±0.34 2.01±0.29 2.45±0.37 2.39±0.19
[5] Meneghetti Simon M. Plentz, Meneghtti Mario R., Wolf Carlos R.,
Silva Eid C., Lima Gilvan E. S., Laelson de Lira Silva, Tatiana M. Serra, Cauduro Fernanda and Lenise G. de Oliviera, “Biodiesel from Castor Oil: A Comparison of Ethanolysis versus Methanolysis”, Energy and Fuels vol. 20, pp. 2262-2265, 2006.
[6] Da Silva Nivea de Lima, Batistella Cesar Benedito, Filho Rubens
Maciel and Wolf Maciel Maria Regina, “Biodiesel Production from
2013 jser.org
International Journal of Scientific & Engineering Research Volume 4, Issue3, March-2013 5
ISSN 2229-5518
Castor Oil: Optimization of Alkahne Ethanolysis", Energy Fuels val.
23, pp. 5636-5642, 2009.
[7] Canoira Laureano, Galean Juan Garcia, Alcantara Ramon, Lapuerta Magin and Garcia-Conteras Reyes, "Fatty acid methyl esters (FAMEs) from castor oil: Production process assessment and synergistic effects in its properties", Renewable Energy Article in Press. pp. 1-10,2009.
IJSER lb) 2013
http://www.ijserorq







