International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1028
ISSN 2229-5518
*Department of Chemistry, No:160,Bangalore city college,
Chellekere,Kalyanagar,Bangalore-5150036,Karnataka, India.
*BE 5th Semester RV college of Engineering, Mysore Road,Bangalore-
560059,Karnataka,India
![]()
Ultrasonic velocity (U), and viscosity (η) have been measured for Poly(metha acrylic acid) and poly (vinyl pyrrolidone) in DMSO have been measured over a widw range of compositions and concentrations at 308.15K.The experimental Ultrasonic velocity values
are used to calculate the adiabatic compressibility (β), molar compressibility ( ϕ )Relaxation strength were calculated. The results of the parameters have been discussed in terms of compatibility of the polymer blend at different concentrations.,
![]()
Ultrasonic investigation of polymer blends in Non –Polar solvents is useful
information in understanding the behaviour of polymer blend systems, because intermolecular and intramolecular association, complex formation and related structural changes affect the compatibility of the system which in turn produces corresponding
variation in the ultrasonic velocity. During the last two decades, considerable studies have been carried out to investigate the compatibility of polymer blends (1).Hourston and Huges (2) and Kuleznev (3) have suggested the use of ultrasonic measurments for investigation of compatibility of polymer blends. Paladhi and Singh (4) have pointed that ultrasonic measure of polymer blends are discussed in terms of miscibility to the degree and nature of the polymer blend concentration. Varadarajulu et al (5) have used ultrasonic interferometer technique for the study of compatibility of the polymers.Due to the complex molecular structure of polymers ,cost and time the direct study is somewhat difficult. Therefore, the useful approach is to study simple ,low cost and rapid techniques such as ultrasonic velocity studies ,viscosity measurements etc are identified . Most of the inter polymer complexes of PMAA with PEO and PVP are studied. The complexes of PMAA net work have been suggested to be used as carriers of enzyme s for the controlled release of drugs. Because of the important applications of PMAA –PVP blends ,the authors have studied the ultrasonic velocity studies of this blend at 308.15K in DMSO which is in turn considered as an industrially important solvent and the results are presented in this paper.
,Mumbai,India Mv =30000) and PVP (M/S SISCO laboratories, Mumbai,India,M v=40000) have been employed in the present study. The total weight of the two components in the solution is always maintained at 2 and 4 g/dl.Ultrasonic velocity measurements were made
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1029
ISSN 2229-5518
using Mittal Enterprises(new Delhi)Ultrasonic Interferometer operating at 2 MHz frequency with an accuracy of +/- 0.2% as described earlier (6).The temperature was maintained constant by circulating water from a thermostat with athermal stability of +/-0.050C through a double walled jacket of ultrasonic experimental cell.
The measured values of ultrasonic velocity (v) and the various acoustical and thermo dynamical parameters are calculated from the measured data such as
Adiabatic Compressibility (β) ,Molar compressibility ( ϕ )Relaxation strength using the relations reported by the Chowdojirao etal (6)in their publication and are presented in Fig.3 (a,b&c)
System
ISoluJbility
SIEnteraction ParaRmeters
Table:2 Interaction parameters of Polymer- Solvent and polymer blend solvent systems
IJSER © 2013 http://www.ijser.org
'll!!ia)
; Research, Volume 4, Issue 12, December-2013
1030
![]()
![]()
r15e-/ i
::j
,.:K-·----·--... =::!=---
'-. - ---....-- - - -
4_ j
0 20
-- - --- -
40 60
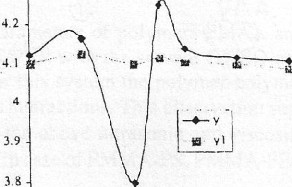
- - -- - -·.,
ghtpercentage of PMAA ( BO 100
component 1)
Fig. 1 Ultrasonic Vela .
IPEfl:enrage of PMAA ( City {v) Vs weigh(
RcM-PVA blends I c mponent 1) in
CQI'Icemrauons In O S(y) & 4% (y1)
0.147 J
0.139
3.7------,-·---.- ----.,--·- - --, ·-··-- ---,
0 20 40 60 80 100
Weight pP.rcentage olPMAA (component 1)
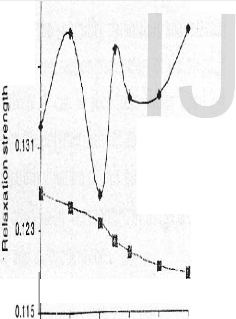

Fig.3(b) Adiabatic compressibilityVs weight percentage of PMAA (component 1) in PMAA ·PVP blends ot 2% (y) & 4% (y1) concentrations in DMSO
![]()
r
o o o oo so
Wei rc nlaeol rMAA ( omonenl1)
rl . (a) Relaxa o Sire (rs) Vs
l nl ercen!ae ol PMAA
(c neol1)inPMAA·PVP olens or%( l & 4% (y1) concenlmlions in OMSO
IJSER 2013
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1031
ISSN 2229-5518
The variations of ultrasonic velocity (v) with increasing percentage of PMAA (component1) in the blend has been plotted in Fig1. It is seen from Fig.1 that for 2 % concentration the ultrasonic velocity varies non-linearly with the composition but varies linearly for 4% concentration. This behaviour indicates that PMAA and PVP blend exhibits immiscibility at lower concentration but miscibility at higher concentration..The more information on the behaviour of the blend system the variations of Relaxation strength and adiabatic compressibility ( ϕ ) with compositions of 2% and 4% are plotted as shown in Fig 3 .These parameters also varies non-linearly with the 2% composition but varies linearly for 4% .This clearly indicates that at higher concentrations the blend is showing miscibility and at lower concentrations it exhibits immiscibility in DMSO .The same trend has been reported by Sidkey et.al (7).
The structural changes influence the ultrasonic velocity to a marked extent as compared to compressibility. From Table-1& 2, it is observed that the values of ultrasonic velocity values increases with increase in blend concentration. This increasing trend indicates the existence of blend -solvent interaction occurring in these systems.
From ultrasonic velocity measurements the blend PMAA-PVP is found to be incompatible at lower concentrations (2%) and compatible at higher concentration (4%) in DMSO.which also concluded that blend -solvent interactions are dominating .
[1] S.Krasue, In : ‘polymer blends’ (edited by DR Paul and S.Newman),Vol 1,Academic press, New York (1978)
[2] D.J.Hourston and I.D. Hughes ,Polymer, 19,(1978)1181
[3] V.N. Kuleznev, D.L Meilnikova and V.D. Klykova , Eur.Polm.J.,14 ,(1978) 455. [4] R.Paladhi and R.P.Singh , Eur.Polymk. J,. 30 (1994) 251.
[5] K.ChowdojiRao, A.Varadarajulu, and S.V.Venkata Naidu, Acta Polymeric ,40 (12)
(1989) 743.
[6] A.Varadarajulu, R.Lakshminarayana Reddy , S.M. Raghavendra and S.Akheel Ahemad
Eur.Polym.J,., 34 (1998)1
[7]M.A. Sidkey,A.M. Abd El Fattah and N.S. Abd El ali ,J. of Applied plymer science, 46 (1992) 581.
IJSER © 2013 http://www.ijser.org